Automated DWI-FLAIR mismatch assessment in stroke using DWI only
- PMID: 40874815
- PMCID: PMC12394211
- DOI: 10.1177/23969873251362712
Automated DWI-FLAIR mismatch assessment in stroke using DWI only
Abstract
Introduction: In Acute Ischemic Stroke (AIS), mismatch between Diffusion-Weighted Imaging (DWI) and Fluid-Attenuated Inversion-Recovery (FLAIR) helps identify patients who can benefit from thrombolysis when stroke onset time is unknown (15% of AIS). However, visual assessment has suboptimal observer agreement. Our study aims to develop and validate a Deep-Learning model for predicting DWI-FLAIR mismatch using solely DWI data.
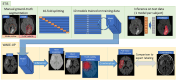
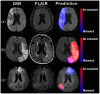
Patients and methods: This retrospective study included AIS patients from ETIS registry (derivation cohort, 2018-2024) and WAKE-UP trial (validation cohort, 2012-2017). DWI-FLAIR mismatch was rated visually. We trained a model to predict manually-labeled FLAIR visible areas (FVA) matching the DWI lesion on baseline and early follow-up MRIs, using only DWI as input. FVA-index was defined as the volume of predicted regions. Area under the ROC curve (AUC) and optimal FVA-index cutoff to predict DWI-FLAIR mismatch in the derivation cohort were computed. Validation was performed using baseline MRIs of the validation cohort.
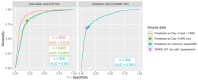
Results: The derivation cohort included 3605 MRIs in 2922 patients and the validation cohort 844 MRIs in 844 patients. FVA-index demonstrated strong predictive value for DWI-FLAIR mismatch in baseline MRIs from the derivation (n = 2453, AUC = 0.85, 95%CI: 0.84-0.87) and validation cohort (n = 844, AUC = 0.86, 95%CI: 0.84-0.89). With an optimal FVA-index cutoff at 0.5, we obtained a kappa of 0.54 (95%CI: 0.48-0.59), 70% sensitivity (378/537, 95%CI: 66-74%) and 88% specificity (269/307, 95%CI: 83-91%) in the validation cohort.
Discussion and conclusion: The model accurately predicts DWI-FLAIR mismatch in AIS patients with unknown stroke onset. It could aid readers when visual rating is challenging, or FLAIR unavailable.
Keywords: Ischemic stroke; artificial intelligence; decision support techniques; diffusion magnetic resonance imaging; magnetic resonance imaging.
Conflict of interest statement
J.B.F reports consulting and advisory board fees from AbbVie, AC Immune, Alzheon, Artemida, BioClinica/Clario, Biogen, Bristol Myers Squibb, Brainomix, Cerevast, C2N Diagnostics, Daiichi-Sankyo, EISAI, Eli Lilly, F. Hoffmann-LaRoche AG, GlaxoSmithKline, Guerbet, Ionis Pharmaceuticals, IQVIA, Janssen, Julius Clinical, jung diagnostics, Lantheus Medical Imaging, Merck, Novo Nordisk, Octapharma AG, Premier Research, ProPharma Group, Prothena Biosciences, Regeneron Pharmaceuticals, Roche, Syneos, Tau Rx, Vertex Pharmaceuticals, and Worldwide Clinical Trials outside the submitted work.
G.Th reports personal consulting fees from Acandis, personal consulting fees from Astra Zeneca, personal consulting fees from Bayer, personal consulting fees from Boehringer Ingelheim, personal consulting fees from Stryker, personal payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from: Acandis, Alexion, Amarin, Bayer, Boehringer Ingelheim, BristolMyersSquibb/Pfizer, Daiichi Sankyo, Stryker, outside the submitted work.
C.Z.S is supported by a research grant from Health Research Foundation of Central Denmark Region. Also reports speaker fee from Pfizer.
V.T. reports consulting fees from Bayer, Boehringer Ingelheim, Medtronic.
C.G. declares, independent of the presented study, grants from Deutsche Forschungsgemeinschaft (DFG), Deutsches Zentrum f. Luft- und Raumfahrt (DLR), Hertie Foundation, Wegener Foundation, Schilling Foundation, Werner Otto Foundation, Merz Pharmaceuticals, Allergan, European Union; CG declares consulting fees from AlphaSights Ltd. and Life Science Praxis S.L., honoraria (for lectures, presentations) from AstraZeneca GmbH, Elements Communications Ltd., Boehringer Ingelheim, Streamedup GmbH, Abbott Medical, Bayer AG; CG declares participation in the DSMB of RESSTORE1, work as an editor of INFO Neurologie & Psychiatrie, Therapie und Verlauf neurologischer Erkrankungen (Textbook), and membership of the presidium of the German Neurological Society (DGN).
K.W.M. reports consultancy fees from Boehringer Ingelheim, Abbvie, Lumosa, Hyperfine; Lecture Fees – Boehringer Ingelheim, Brainomix, IschemaView; Trial Support – Boehringer Ingelheim (drug supply for ATTEST-2).
M.E. reports grants from Bayer and fees paid to the Charité from Amgen, AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, BMS, Daiichi Sankyo, Sanofi, Pfizer, all outside the submitted work.
R.L. reports compensation from iSchemaView for other services.
All other authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


References
-
- Pu L, Wang L, Zhang R, et al. Projected global trends in ischemic stroke incidence, deaths and disability-adjusted life years from 2020 to 2030. Stroke 2023; 54: 1330–1339. - PubMed
-
- Saver JL. Time is brain-quantified. Stroke 2006; 37: 263–266. - PubMed
-
- Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359: 1317–1329. - PubMed
-
- Thomalla G, Cheng B, Ebinger M, et al. DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4·5 h of symptom onset (PRE-FLAIR): a multicentre observational study. Lancet Neurol 2011; 10: 978–986. - PubMed
LinkOut - more resources
Full Text Sources

