A smooth muscle cell lncRNA controls angiogenesis in chronic limb-threatening ischemia through miR-143-3p/HHIP signaling
- PMID: 40875440
- PMCID: PMC12520679
- DOI: 10.1172/JCI188559
A smooth muscle cell lncRNA controls angiogenesis in chronic limb-threatening ischemia through miR-143-3p/HHIP signaling
Abstract
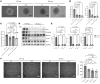
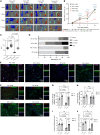
Peripheral artery disease (PAD) often advances to chronic limb-threatening ischemia (CLTI), resulting in severe complications such as limb amputation. Despite the potential of therapeutic angiogenesis, the mechanisms of cell-cell communication and transcriptional changes driving PAD are not fully understood. Profiling long noncoding RNAs (lncRNAs) from gastrocnemius muscles of participants with or without CLTI revealed that a vascular smooth muscle cell-enriched (SMC-enriched) lncRNA, CARMN, was reduced with CLTI. This study explored how a SMC lncRNA-miRNA signaling axis regulates angiogenesis in limb ischemia. CARMN-KO mice exhibited reduced capillary density and impaired blood flow recovery and tissue necrosis following limb ischemia. We found that CARMN-KO SMC supernatants inhibited endothelial cell (EC) proliferation, spheroid sprouting, and network formation. RNA-seq identified downregulation of the Hedgehog signaling pathway in CARMN-KO models and revealed that CARMN regulates this pathway through its downstream miRNA, miR-143-3p, which targets Hedgehog-interacting protein (HHIP), an antagonist of Hedgehog signaling. Delivery of HHIP-specific siRNA or miR-143-3p mimics rescued EC angiogenic defects and improved blood flow recovery in both CARMN-KO and WT mice. These findings underscore the critical role of CARMN in modulating angiogenesis through the miR-143-3p-HHIP-Hedgehog signaling axis, providing insights into SMC-EC interactions and potential therapeutic strategies for CLTI.
Keywords: Angiogenesis; Cardiovascular disease; Endothelial cells; Noncoding RNAs; Vascular biology.
Figures




Comment in
- CARMN orchestrates angiogenesis from behind the opera scenes: signing love letters to the endothelium doi: 10.1172/JCI197708
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

