Mapping disease-specific vascular cell populations responsible for obliterative arterial remodelling during the development of pulmonary arterial hypertension
- PMID: 40875786
- PMCID: PMC12560791
- DOI: 10.1093/cvr/cvaf146
Mapping disease-specific vascular cell populations responsible for obliterative arterial remodelling during the development of pulmonary arterial hypertension
Abstract
Aims: Pulmonary arterial hypertension (PAH) is a lethal pulmonary vascular disease characterized by arteriolar pruning and occlusive vascular remodelling leading to increased pulmonary vascular resistance and eventually right heart failure. While endothelial cell (EC) injury and apoptosis are known triggers for this disease, the mechanisms by which they lead to complex arterial remodelling remain obscure. We employed multiplexed single-cell RNA sequencing at multiple timepoints during the onset and progression of disease in a model of severe PAH to identify mechanisms involved in the development of occlusive arterial lesions.
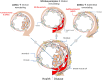
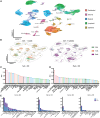
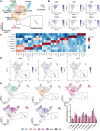
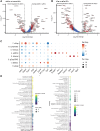
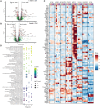
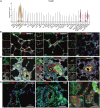
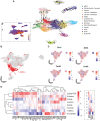
Methods and results: Single-cell transcriptional analysis resolved 44 global lung cell populations, with widespread early transcriptomic changes at 1 week affecting endothelial, stromal, and immune cell populations. In particular, two EC clusters were greatly expanded during PAH development and were identified as being disease specific: a relatively de-differentiated (dD) EC population that was enriched for Cd74 expression while exhibiting a loss of endothelial identity; and an activated arterial EC (aAEC) population that uniquely exhibited persistent differential gene expression throughout PAH development consistent with a growth regulated state. dDECs were primed to undergo endothelial-mesenchymal transition as evidenced by reduced activity of master EC transcription factors, Erg and Fli1, and further supported by RNA velocity analysis showing vectors leading to fibroblast clusters. Of note, aAECs exhibited high expression of Tm4sf1, a gene implicated in cancer cell growth, that was also expressed by a smooth muscle (SM)-like pericyte cluster, and were highly localized to regions of arterial remodelling in both the rat model and PAH patients, contributing to intimal occlusive lesions and SM-like pericytes forming bands of medial muscularization.
Conclusion: Together these findings implicate disease-specific vascular cells in PAH progression and suggest that TM4SF1 may be a novel therapeutic target for arterial remodelling.
Keywords: Endothelial cells; Pericytes; Pulmonary arterial hypertension; Vascular remodelling.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: none declared.
Figures


Comment in
-
The devil is in the details: addressing lung cell heterogeneity in sugen-hypoxia rats and its relevance to pulmonary arterial hypertension.Cardiovasc Res. 2025 Oct 28;121(13):1981-1982. doi: 10.1093/cvr/cvaf188. Cardiovasc Res. 2025. PMID: 41071933 No abstract available.
References
-
- Hoeper MM, Badesch DB, Ghofrani HA, Gibbs JSR, Gomberg-Maitland M, McLaughlin VV, Preston IR, Souza R, Waxman AB, Grünig E, Kopeć G, Meyer G, Olsson KM, Rosenkranz S, Xu Y, Miller B, Fowler M, Butler J, Koglin J, de Oliveira Pena J, Humbert M. Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension. N Engl J Med 2023;388:1478–1490. - PubMed
-
- Cober ND, VandenBroek MM, Ormiston ML, Stewart DJ. Evolving concepts in endothelial pathobiology of pulmonary arterial hypertension. Hypertension 2022;79:1580–1590. - PubMed
-
- Schupp JC, Adams TS, Cosme C, Raredon MSB, Yuan Y, Omote N, Poli S, Chioccioli M, Rose K-A, Manning EP, Sauler M, DeIuliis G, Ahangari F, Neumark N, Habermann AC, Gutierrez AJ, Bui LT, Lafyatis R, Pierce RW, Meyer KB, Nawijn MC, Teichmann SA, Banovich NE, Kropski JA, Niklason LE, Pe’er D, Yan X, Homer RJ, Rosas IO, Kaminski N. Integrated single-cell atlas of endothelial cells of the human lung. Circulation 2021;144:286–302. - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

