Development and Characterization of a Murine Lung Adenocarcinoma Cell Line With High Thoracic Pleural Metastatic Potential
- PMID: 40877194
- PMCID: PMC12396039
- DOI: 10.21873/invivo.14067
Development and Characterization of a Murine Lung Adenocarcinoma Cell Line With High Thoracic Pleural Metastatic Potential
Abstract
Background/aim: Pleural metastasis and malignant pleural effusion (MPE) are common complications of lung adenocarcinoma. Patients with MPE have poor outcomes, with overall survival ranging from 5 to 11.4 months. The lack of established cell lines and stable animal models of pleural metastasis has limited studies on the underlying mechanisms of MPE development. In this study, we aimed to develop a murine lung adenocarcinoma cell line with high thoracic pleural metastatic potential.
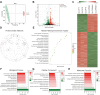
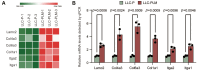
Materials and methods: Luciferase-tagged Lewis lung carcinoma (LLC) cells were implanted into the pleural cavity of C57bl/6 mice, with five rounds of subsequent extraction from pleural foci and reinjection into the pleural cavity. The metastatic properties of the established cell line were verified in vivo by evaluating the metastatic burden and MPE volume (n=5). In vitro, the metastatic ability of cell lines was assessed by scratch assay, transwell migration assay, cell-matrix adhesion assay and cell-cell adhesion assay (3-5 replicates). The transcription profile was characterized by mRNA sequencing. Differential analysis and KEGG enrichment were performed to show their distinctions. Differential genes were verified by quantitative real-time PCR (qPCR).
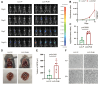
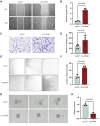
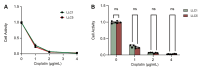
Results: An LLC subpopulation with high thoracic pleural metastatic potential was generated and named LLC-PLM. In vivo, compared with parental LLC (LLC-P), LLC-PLM demonstrated a greater incidence of MPE and greater MPE volumes. In vitro, LLC-PLM demonstrated increased metastatic capacity and augmented adhesion capacities, compared to LLC-P. Transcriptomic analysis revealed that pathways related to adhesion, migration, and membrane signaling were notably enriched and activated in LLC-PLM cells. Relative genes were obviously activated, including Lamc2, Col4a3, Col6a3, Col1a1, Itga2 and Itga1.
Conclusion: We successfully established a murine cell line LLC-PLM that can serve as a valuable tool for studying pleural metastasis and MPE.
Keywords: Lewis lung carcinoma (LLC); Lung adenocarcinoma; malignant pleural effusion (MPE); murine tumor model; pleural metastasis.
Copyright © 2025, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
The Authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Similar articles
-
Effect of intrapleural anti-Vascular Endothelial Growth Factor (VEGF) associated with nab paclitaxel in a murine model of malignant pleural effusion.BMC Cancer. 2025 Aug 2;25(1):1261. doi: 10.1186/s12885-025-14622-x. BMC Cancer. 2025. PMID: 40753384 Free PMC article.
-
Caveolin-1 inhibits the proliferation and invasion of lung adenocarcinoma via EGFR degradation.Sci Rep. 2025 Jul 1;15(1):21654. doi: 10.1038/s41598-025-05259-8. Sci Rep. 2025. PMID: 40594106 Free PMC article.
-
Glucagon-like peptide-1 receptor agonists inhibit the progression of malignant pleural effusion in obese mice by regulating the metabolism of pleural effusion.J Transl Med. 2025 Aug 8;23(1):886. doi: 10.1186/s12967-025-06875-8. J Transl Med. 2025. PMID: 40781724 Free PMC article.
-
Interventions for the management of malignant pleural effusions: a network meta-analysis.Cochrane Database Syst Rev. 2016 May 8;2016(5):CD010529. doi: 10.1002/14651858.CD010529.pub2. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2020 Apr 21;4:CD010529. doi: 10.1002/14651858.CD010529.pub3. PMID: 27155783 Free PMC article. Updated.
-
A systematic review of evidence on malignant spinal metastases: natural history and technologies for identifying patients at high risk of vertebral fracture and spinal cord compression.Health Technol Assess. 2013 Sep;17(42):1-274. doi: 10.3310/hta17420. Health Technol Assess. 2013. PMID: 24070110 Free PMC article.
References
-
- Detterbeck FC, Bolejack V, Arenberg DA, Crowley J, Donington JS, Franklin WA, Girard N, Marom EM, Mazzone PJ, Nicholson AG, Rusch VW, Tanoue LT, Travis WD, Asamura H, Rami-Porta R, IASLC Staging and Prognostic Factors Committee, Advisory Boards, Multiple Pulmonary Sites Workgroup, Participating Institutions The IASLC Lung Cancer Staging Project: Background data and proposals for the classification of lung cancer with separate tumor nodules in the forthcoming eighth edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2016;11(5):681–692. doi: 10.1016/j.jtho.2015.12.114. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
