DJ-1 counteracts Caveolin-1-mediated necroptosis to inhibit epithelial barrier dysfunction in colitis
- PMID: 40877233
- PMCID: PMC12394565
- DOI: 10.1038/s41419-025-07989-z
DJ-1 counteracts Caveolin-1-mediated necroptosis to inhibit epithelial barrier dysfunction in colitis
Abstract
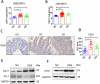
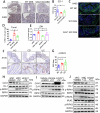
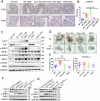
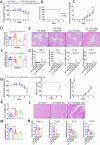
Caveolin-1 (CAV1), a pivotal protein implicated in endothelial cell-mediated angiogenesis, assumes an ambiguous role with elusive underlying mechanisms in the pathogenesis of inflammatory bowel disease (IBD). In this investigation, we delineated the involvement of CAV1 in murine models of dextran sulfate sodium (DSS)-induced colitis. CAV1 knockout mice manifested attenuated pathological and inflammatory damage to the epithelium, whereas mice overexpressing CAV1 exhibited contrasting outcomes. In vivo, the accumulation of epithelial CAV1 contributed to the disruption of the epithelial barrier by promoting necroptosis. Subsequent mechanistic analyses revealed that the colitis-protective protein DJ-1 regulated CAV1 through a proteasome-mediated protein degradation pathway. Utilizing necroptosis-modeled organoids from murine intestines and pharmacological inhibition of necroptosis, our findings demonstrated that the DJ-1/CAV1 pathway governed epithelial inflammation via necroptosis in the context of colitis. In summary, our research revealed that epithelial CAV1 aggravated necroptosis in experimental colitis, leading to impairment of the epithelial barrier, which was negatively regulated by DJ-1.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval: All animal experiments were performed according to guidelines approved by the laboratory animal welfare and ethics review committee of Zhejiang University (approval No. ZJU20250339). And all clinical researches were approved by the ethical committee of the Fourth Affiliated Hospital Zhejiang University School of Medicine (approval No. K2025080).
Figures



References
-
- Kobayashi T, Siegmund B, Le Berre C, Wei SC, Ferrante M, Shen B, et al. Ulcerative colitis. Nat Rev Dis Prim. 2020;6:74. - PubMed
-
- Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42. - PubMed
-
- Peyrin-Biroulet L, Loftus EV Jr., Colombel JF, Sandborn WJ. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol. 2010;105:289–97. - PubMed
-
- Schroeder F, Huang H, McIntosh AL, Atshaves BP, Martin GG, Kier AB. Caveolin, sterol carrier protein-2, membrane cholesterol-rich microdomains and intracellular cholesterol trafficking. Sub-Cell Biochem. 2010;51:279–318. - PubMed
-
- Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiological Rev. 2004;84:1341–79. - PubMed
MeSH terms
Substances
Grants and funding
- 82030019/National Natural Science Foundation of China (National Science Foundation of China)
- 82300582/National Natural Science Foundation of China (National Science Foundation of China)
- 82100541/National Natural Science Foundation of China (National Science Foundation of China)
- 2023M743027/China Postdoctoral Science Foundation
- 2024T170778/China Postdoctoral Science Foundation
LinkOut - more resources
Full Text Sources

