Epigenetically conferred ring-stage survival in Plasmodium falciparum against artemisinin treatment
- PMID: 40877256
- PMCID: PMC12394585
- DOI: 10.1038/s41467-025-62479-2
Epigenetically conferred ring-stage survival in Plasmodium falciparum against artemisinin treatment
Abstract
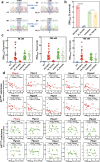
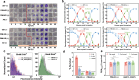
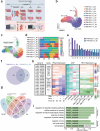
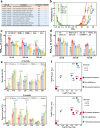
Artemisinin and its semisynthetic derivatives (ART) are crucial medicines in artemisinin-based combination therapies worldwide. Despite ART's efficacy, small proportions of young intraerythrocytic ring stage parasites can survive the drug's short half-life, and dormant forms can cause recrudescence if not cleared by partner drugs. Certain mutations in the Kelch propeller region of P. falciparum protein (PfK13) are linked to the higher ring-stage survival (RS), which above 1% can be a feature of 'artemisinin partial resistance'. Emerging evidence indicates epigenetic modulators may contribute to RS. Here, we report systematic evaluations of all putative histone acetyltransferases (HATs) of P. falciparum in 30 culture-adapted field isolates and 43 subcloned field isolates. Only PfMYST shows a full association with RS phenotype modulations. Knockdown experiments confirm the linkage of Pfmyst expression to these modulations, with evidence of altered metabolic processes. Through single-cell RNA sequencing, ChIP-seq analysis, and CRISPR/cas9 genetic manipulation, PfMYST-targeted RS-related genes have been identified and functionally validated. Multi-omics analysis indicates significant interplay of PfMYST and PfK13 mechanisms in RS. PfMYST epigenetic modulation extends to other antimalarials, including amodiaquine, pyrimethamine, chloroquine, and pyronaridine. Collectively, our findings provide important information on the epigenetic regulatory mechanism of P. falciparum RS after pulses of ART and other antimalarials.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Li, G. Q., Arnold, K., Guo, X. B., Jian, H. X. & Fu, L. C. Randomised comparative study of mefloquine, qinghaosu, and pyrimethamine-sulfadoxine in patients with falciparum malaria. Lancet2, 1360–1361 (1984). - PubMed
-
- Giao, P. T. et al. Artemisinin for treatment of uncomplicated falciparum malaria: is there a place for monotherapy? Am. J. Tropical Med. Hyg.65, 690–695 (2001). - PubMed
-
- World Health Organization. Chemotherapy of malaria: report of a WHO scientific group. In World Health Organization Technical Report Series No. 375. (1967).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

