A comparative analysis of heterogeneity in lung cancer screening effectiveness in two randomised controlled trials
- PMID: 40877276
- PMCID: PMC12394595
- DOI: 10.1038/s41467-025-63471-6
A comparative analysis of heterogeneity in lung cancer screening effectiveness in two randomised controlled trials
Abstract
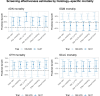
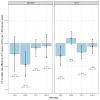
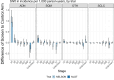
Clinical trials demonstrate that screening can reduce lung cancer mortality by over 20%. However, lung cancer screening effectiveness (reduction in lung cancer specific mortality) may vary by personal risk-factors. Here we evaluate heterogeneity in lung cancer screening effectiveness through traditional sub-group analyses, predictive modelling approaches and machine-learning in individual-level data from the Dutch-Belgian lung cancer screening trial (NELSON; 14,808 participants, 12,429 men, 2377 women, 2 persons with an unknown sex) and the National Lung Screening Trial (NLST; 53,405 participants, 31,501 men, 21,904 women). We find that screening effectiveness varies by pack-years (screening effectiveness ranges across trials: lowest groups = 26.8-50.9%, highest groups = 5.5-9.5%), smoking status (screening effectiveness ranges across trials: former smokers = 37.8-39.1%, current smokers = 16.1-22.7%) and sex (screening effectiveness ranges across trials: women = 24.6-25.3%; men = 8.3-24.9%). Furthermore, screening effectiveness varies by histology (screening effectiveness ranges across trials: adenocarcinoma = 17.8-23.0%, other lung cancers = 24.5-35.5%, small-cell carcinoma = 9.7%-11.3%). Screening is ineffective for squamous-cell carcinoma in NLST (screening effectiveness = 27.9% (95% confidence interval: 69.8% increase to 4.5% decrease) mortality increase) but effective in NELSON (screening effectiveness = 52.2% (95% confidence interval: 25.7-69.1% decrease) mortality reduction). We find that variations in screening effectiveness across pack-years, smoking status, and sex are primarily explained by a greater prevalence of histologies with favourable screening effectiveness in these groups. Our study shows that heterogeneity in lung screening effectiveness is primarily driven by histology and that relaxing smoking-related screening eligibility criteria may enhance screening effectiveness.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: C.M.v.d.A. declares roles in the WHO-IARC European Code Against Cancer working group, B3care user committee and Expert Mission Cancer Screening in Georgia outside of the submitted work. H.J.G. declares consulting fees from Eli Lilly outside of the submitted work. P.A.d.J. research support from Philips Healthcare to their institute outside of the submitted work. J.A. declares speakers fees from Eli Lilly, MSD and BIOCAD, patents from Pamgene and Amphara, data safety monitoring/advisory board participation for Eli-Lilly, Amphara, BIOCAD and MSD, boardmembership of the IASLC and stock ownership in Amphera outside of the submitted work. H.J.d.K. declares consulting fees from Bayer and honoraria from TEVA/Menarini/Astra Zeneca outside of the submitted work. K.t.H. declares grants from NIH, Horizon 2020, University of Zurich, Cancer Research UK, Cancer Australia and the Australian Ministry of Health, speakers fees from Johnson&Johnson and Centre Hospitalier Universitaire Vaudois paid to their institute and travel support from the Rescue Lung Society outside of the submitted work. The remaining authors declare no competing interests.
Figures


References
-
- de Koning, H. J. et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N. Engl. J. Med.382, 503–513 (2020). - PubMed
-
- Becker, N. et al. Lung cancer mortality reduction by LDCT screening-Results from the randomized German LUSI trial. Int. J. Cancer146, 1503–1513 (2020). - PubMed
-
- Mathilde, M. W. W. et al. Results of the randomized Danish lung cancer screening trial with focus on high-risk profiling. Am. J. Respir. Crit. Care Med.193, 542–551 (2016). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

