A comparison of oral microbiome composition between highly trained competitive athletes and untrained controls
- PMID: 40877538
- PMCID: PMC12394404
- DOI: 10.1038/s41598-025-16835-3
A comparison of oral microbiome composition between highly trained competitive athletes and untrained controls
Abstract
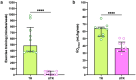
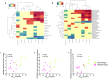
The oral microbiome has a crucial role in nitric oxide (NO) production and contributes to oral and systemic health. This study compared oral microbiome composition and markers of NO production between highlytrained competitive athletes and inactive controls. Competitive athletes and untrained controls (N = 10 per group) were recruited. Saliva, plasma, supragingival plaque and the tongue dorsum microbiome were sampled. The microbiome was examined using long-read 16S rRNA sequencing and ozone-based chemiluminescence used to measure nitrate (NO3-) and nitrite (NO2-) levels. Weekly training duration was recorded and aerobic fitness capacity (V̇O2max) assessed via maximal exercise testing.The beta-diversity of the tongue dorsum microbiome differed between groups (Adonis p = 0.046) and athletes had a higher relative abundance of NO3--reducing Rothia mucilaginosa and unclassified Gemella species. No significant differences were detected in the supragingival plaque. Positive correlations were detected between R. mucilaginosa and unclassified Gemella species and aerobic fitness. Athletes had higher levels of salivary NO3- (p = 0.003) and NO2- (p = 0.03). Exercise training may impact the tongue dorsum microbiome more than supragingival plaque, with the relative abundance of specific health-associated bacteria higher in the tongue dorsum microbiome of athletes. The robust methodologies employed in this study highlight a possible link between consistent exercise and the development of an oral microbiome conducive to health. However, further research is needed to explore the mechanisms connecting exercise, the oral microbiome, and overall health.
Keywords: 16 s rRNA; Dental disease; Exercise; Exercise training; Nitrate; Nitrate-reduction; Nitric oxide; Nitrite-production; Oral microbiome; Physical activity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: A.M. and B.T. Rosier are coinventors in a pending patent application owned by the FISABIO Institute, which protects the use of NO3- as a prebiotic and certain NO3–reducing bacteria as probiotics. The remaining authors declare no competing interests. Ethical approval: The study was approved by the School of Health and Life Sciences Research Ethics Committee at the University of the West of Scotland (submission number 14970). With the exception of pre-recruitment trial registration, all procedures were carried out in accordance with the World Medical Association Declaration of Helsinki. All participants gave written informed consent.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

