Cardioprotective effects of extracellular vesicles from hypoxia-preconditioned mesenchymal stromal cells in experimental pulmonary arterial hypertension
- PMID: 40877897
- PMCID: PMC12395730
- DOI: 10.1186/s13287-025-04604-y
Cardioprotective effects of extracellular vesicles from hypoxia-preconditioned mesenchymal stromal cells in experimental pulmonary arterial hypertension
Abstract
Background: During pulmonary arterial hypertension (PAH), cardiac cells develop a hypertrophic and apoptosis-resistant phenotype. Mesenchymal stromal cell (MSC) therapy has been shown to mitigate pulmonary vascular remodeling in PAH; however, successful application is limited by low potency and the need for a high number of MSCs. MSCs exposed to hypoxia release more extracellular vesicles (EV)s with different content than normoxia. We aimed to evaluate the proteomic profile and therapeutic effects of EVs derived from normoxia- and hypoxia-preconditioned MSCs on cardiac tissue remodeling in experimental PAH.
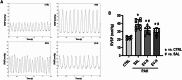
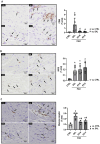
Methods: Isolated bone marrow MSCs were subjected to normoxia (N, 21%O2) or hypoxia (H, 1%O2) for 48 h and EVs were collected from the MSCs by ultracentrifugation. Proteomic data of the EVs were reanalyzed using PatternLab for Proteomics 5.0. Thirty-two male Wistar rats were randomly assigned to PAH plus intraperitoneal monocrotaline (60 mg/kg) or control (CTRL) with saline. On day 14, PAH animals received saline (1 mL/kg; PAH-SAL), EV-N (EVs from 1 × 106 MSCs; PAH-EV-N) or EV-H (EVs from 1 × 106 MSCs; PAH-EV-H) by jugular vein. On day 28, right ventricular systolic pressure (RVSP), pulmonary acceleration time/pulmonary ejection time (PAT/PET) ratio, right ventricle (RV) outflow diameter, and right ventricular hypertrophy (RVH) index were evaluated. The heart was harvested for histologic and molecular biology analyses.
Results: Among 695 proteins identified, 203 were present only in EV-H and 51 in EV-N. EV-H was enriched in proteins involved in the negative regulation of mitogen-activated protein kinase and apoptosis pathways. On day 28, both EV-N and EV-H therapies decreased RVSP compared with PAH-SAL (32 ± 5 mmHg and 29 ± 4 mmHg versus 39 ± 2 mmHg; p < 0.01). Only EV-H increased PAT/PET, reduced RV outflow diameter, and the RVH index compared with PAH-SAL. The expressions of c-Myc, a marker of myocardial injury, and p-GSK3β-Ser9, a proliferative marker, were higher in the PAH-SAL group than in the CTRL group. EV-N and EV-H decreased c-Myc expression, but only EV-H significantly reduced p-GSK3β-Ser9.
Conclusion: EV-N and EV-H reduced RVSP, but only EV-H improved RVH and RV outflow diameter, increased the PAT/PET ratio, and downregulated GSK3β protein levels. EVs from hypoxia-preconditioned MSCs demonstrated greater cardioprotective effects than those from normoxia-conditioned MSCs.
Keywords: Cardiac remodeling; Extracellular vesicles; Hypoxic pre-conditioning; Mass spectrometry; Mesenchymal stromal cells; Monocrotaline; Proteomic profile; Ventricle hypertrophy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interest: The authors have no competing interests in the information described in this article.
Figures


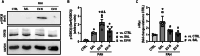


References
-
- Lv X, Li J, Wei R, Meng L, Kong X, Wei K, et al. Ethyl pyruvate alleviates pulmonary arterial hypertension via PI3K-Akt signaling. Mol Cell Biochem. 2025;480(2):1045–54. 10.1007/s11010-024-05020-1 - PubMed
-
- Gurbanov E, Shiliang X. The key role of apoptosis in the pathogenesis and treatment of pulmonary hypertension. Eur J Cardiothorac Surg. 2006;30(3):499–507. 10.1016/j.ejcts.2006.05.026 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

