Enriched environment inhibits GLT-1 ubiquitination by downregulating SMURF1 to attenuate ischemic brain injury induced excitotoxicity
- PMID: 40877964
- PMCID: PMC12395883
- DOI: 10.1186/s13578-025-01457-z
Enriched environment inhibits GLT-1 ubiquitination by downregulating SMURF1 to attenuate ischemic brain injury induced excitotoxicity
Abstract
Background: Excitotoxicity induced by glutamate contributes significantly to ischemic brain injury. The role of enriched environment (EE) in promoting neurological recovery post-stroke is well-established, yet its impact on excitotoxicity remains unclear. This study aimed to elucidate the mechanisms through which EE modulates excitotoxicity in ischemic brain injury using in vivo and in vitro experiments.
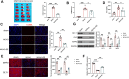
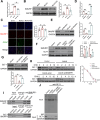
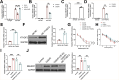
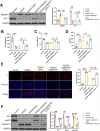
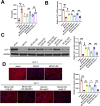
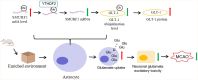
Results: EE exposure effectively reduced ischemic brain injury and glutamate-induced excitotoxicity in middle cerebral artery occlusion (MCAO) rat models. Notably, EE led to upregulated expression of GLT-1, which enhanced glutamate uptake by astrocytes and mitigated cytotoxicity and neuronal death induced by oxygen-glucose deprivation/reperfusion (OGD/R). Furthermore, EE suppressed the upregulation of SMURF1, which interacted with GLT-1 to facilitate its ubiquitin-mediated degradation and downregulate GLT-1 expression. Additionally, EE enhanced m6A modification of SMURF1, promoting YTHDF2-mediated mRNA decay and reducing SMURF1 expression. Knockdown of SMURF1 boosted GLT-1 expression, improving glutamate uptake and alleviating neuronal injury and death caused by OGD/R. Conversely, overexpression of SMURF1 or knockdown of GLT-1 attenuated the neuroprotective effects of EE against excitotoxicity in MCAO rats.
Conclusion: EE mitigates excitotoxicity in ischemic brain injury by inhibiting SMURF1 expression through m6A modification-YTHDF2-dependent pathways, thereby suppressing GLT-1 ubiquitin-mediated degradation and enhancing glutamate uptake.
Supplementary Information: The online version contains supplementary material available at 10.1186/s13578-025-01457-z.
Keywords: Enriched environment; Excitotoxicity; GLT-1; Ischemic brain injury; SMURF1.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experiments were reviewed and approved by the Ethics Committee of the Nanchang University at Nanchang, China (Approval no. CDYFY-IACUC-202310QR019). Consent for publication: Not applicable. Conflict of interest: The authors declare no conflict of interest.
Figures


Similar articles
-
SMURF1 Affects Dental Implant Integration in Diabetes By Regulating PKG2 Expression.Appl Biochem Biotechnol. 2025 Jul 19. doi: 10.1007/s12010-025-05326-w. Online ahead of print. Appl Biochem Biotechnol. 2025. PMID: 40682619
-
Erinacine A attenuates glutamate transporter 1 downregulation and protects against ischemic brain injury.Life Sci. 2022 Oct 1;306:120833. doi: 10.1016/j.lfs.2022.120833. Epub 2022 Jul 23. Life Sci. 2022. PMID: 35882273
-
The Regulation of GLT-1 Degradation Pathway by SIRT4.Neurochem Res. 2023 Sep;48(9):2847-2856. doi: 10.1007/s11064-023-03947-3. Epub 2023 May 13. Neurochem Res. 2023. PMID: 37178383
-
GLT-1 Upregulation as a Potential Therapeutic Target for Ischemic Brain Injury.Curr Pharm Des. 2017;23(33):5045-5055. doi: 10.2174/1381612823666170622103852. Curr Pharm Des. 2017. PMID: 28641538 Review.
-
The role of astrocytic glutamate transporters GLT-1 and GLAST in neurological disorders: Potential targets for neurotherapeutics.Neuropharmacology. 2019 Dec 15;161:107559. doi: 10.1016/j.neuropharm.2019.03.002. Epub 2019 Mar 6. Neuropharmacology. 2019. PMID: 30851309 Free PMC article. Review.
References
-
- Sveinsson OA, Kjartansson O, Valdimarsson EM. Cerebral ischemia/infarction - epidemiology, causes and symptoms. Laeknabladid. 2014;100(5):271–9. - PubMed
-
- Kannan A, Delgardo M, Pennington-FitzGerald W, Jiang EX, Christophe BR, Connolly ES Jr. Pharmacological management of cerebral ischemia in the elderly. Expert Opin Pharmacother. 2021;22(7):897–906. - PubMed
-
- Sakai S, Shichita T. Inflammation and neural repair after ischemic brain injury. Neurochem Int. 2019;130:104316. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

