Targeted soluble epoxide hydrolase inhibits M1 macrophage polarization to improve cartilage injury in temporomandibular joint osteoarthritis
- PMID: 40877980
- PMCID: PMC12395874
- DOI: 10.1186/s12967-025-07003-2
Targeted soluble epoxide hydrolase inhibits M1 macrophage polarization to improve cartilage injury in temporomandibular joint osteoarthritis
Abstract
Background: Macrophage immunomodulation has emerged as a novel intervention and therapeutic strategy for temporomandibular joint osteoarthritis (TMJOA), potentially serving as a key approach for reducing synovial inflammation and promoting cartilage repair. The soluble epoxide hydrolase inhibitor (sEHi), TPPU, has shown potential therapeutic effects against inflammatory diseases and osteogenesis by elevating endogenous Epoxyeicosatrienoic acids (EETs). However, it remains largely unknown whether TPPU can reduce inflammation and cartilage degradation in the TMJOA.
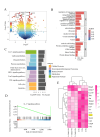
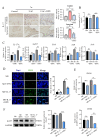
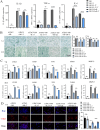
Methods: In vivo, the effects of TPPU on articular cartilage and synovial tissue pathology were assessed using H&E, Masson, Safranin-O/Fast Green staining and immunohistochemistry in a mouse model of TMJOA induced by unilateral anterior crossbite (UAC). RNA-seq and Western Blot was employed to investigate the key signal pathway of TPPU on M1 macrophage polarization. Subsequently, a co-culture system of macrophages and ATDC5 chondrocytes was established, and the influence of TPPU-treated macrophages on chondrogenesis was evaluated through Alcian Blue staining and RT-qPCR.
Results: In vivo, we observed that in UAC-induced TMJOA mice, TPPU significantly reduced the infiltration of inflammatory cells in the synovium and the positive expression of inflammatory factors TNF-α and IL-1β. It also mitigated the degradation of cartilage matrix and increased the positive expression of chondrogenic markers SOX9 and COL II. In vitro experiments revealed that TPPU inhibited the polarization of M1 macrophages, reduced inflammatory responses, and subsequently increased the expression of chondrogenic markers (SOX9 and COLII) in chondrocytes. RNA-seq data indicated that the NF-κB/IL-17 pathway as a putative target following TPPU treatment in macrophages. Further experiments confirmed that the addition of TPPU to macrophages inhibited the reduction in chondrogenesis induced by IL-17 and NF-κB agonists in the co-cultured cells.
Conclusions: Our study elucidates a novel role of TPPU in inhibiting M1 macrophage polarization and modulating inflammatory immune responses via the EETs/NF-κB/IL-17 axis, thereby inhibiting cartilage damage in TMJOA.
Keywords: EETs; IL-17; Macrophage; NF-κB; TMJOA; TPPU.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All procedures were conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Dalian Medical University (Approval No. 2023004). Written informed consent was obtained from all patients. All the animal experiments were approved by the Institutional Animal Care and Use Committee of Dalian Medical University (No. AEE22004). Consent for publication: Consent to publish has been obtained from all authors. Competing interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
Muscone ameliorates osteoarthritis progression by inhibiting M1 macrophages polarization via Nrf2/NF-κB axis and protecting chondrocytes.Toxicol Appl Pharmacol. 2025 Oct;503:117494. doi: 10.1016/j.taap.2025.117494. Epub 2025 Jul 29. Toxicol Appl Pharmacol. 2025. PMID: 40744211
-
The soluble epoxide hydrolase inhibitor TPPU alleviates Aβ-mediated neuroinflammatory responses in Drosophila melanogaster and cellular models of alzheimer's disease.J Inflamm (Lond). 2025 Jun 23;22(1):25. doi: 10.1186/s12950-025-00449-7. J Inflamm (Lond). 2025. PMID: 40551105 Free PMC article.
-
DDX5 Alleviates Temporomandibular Joint Osteoarthritis via TNF-Induced NF-κB Signaling Pathway.Oral Dis. 2025 Jul;31(7):2229-2242. doi: 10.1111/odi.15294. Epub 2025 Feb 27. Oral Dis. 2025. PMID: 40013619
-
Macrophage Reprogramming: Emerging Molecular Therapeutic Strategies for Nephrolithiasis.Biomolecules. 2025 Jul 28;15(8):1090. doi: 10.3390/biom15081090. Biomolecules. 2025. PMID: 40867535 Free PMC article. Review.
-
IL-11-mediated macrophage crosstalk drives renal inflammation and fibrosis: A novel therapeutic target in chronic kidney disease.Life Sci. 2025 Sep 3;380:123949. doi: 10.1016/j.lfs.2025.123949. Online ahead of print. Life Sci. 2025. PMID: 40889697 Review.
References
-
- Manna CA, Costa M, Alves Filho CA, Beneti IM. Isolate or combined use of platelet-rich plasma in the treatment of temporomandibular joint osteoarthritis: a concise systematic review. MedNEXT Journal of Medical and Health Sciences. 2023. 10.54448/mdnt23S206.
-
- Pantoja LLQ, de Toledo IP, Pupo YM, Porporatti AL, De Luca Canto G, Zwir LF, Guerra ENS. Prevalence of degenerative joint disease of the temporomandibular joint: a systematic review. Clin Oral Investig. 2019;23:2475–88. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

