Vancomycin-Induced Acute Kidney Injury in Intensive Care Patients: A Target Trial Emulation Study Using Multicenter Routinely Collected Data
- PMID: 40878006
- PMCID: PMC12394726
- DOI: 10.1002/pds.70205
Vancomycin-Induced Acute Kidney Injury in Intensive Care Patients: A Target Trial Emulation Study Using Multicenter Routinely Collected Data
Abstract
Purpose: The potential of vancomycin to cause acute kidney injury (AKI) in adult intensive care patients is subject to debate due to suboptimal designs of past studies. Therefore, we aimed to estimate the effect of initiating vancomycin versus one of several minimally nephrotoxic alternative antibiotics on the 14-day risk of AKI using the target trial emulation framework.
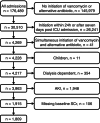
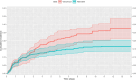
Methods: A hypothetical trial was emulated using routinely collected data from 15 Dutch intensive care units (ICUs) spanning 2010-2019. We used an active comparator control group with the following alternative antibiotics: clindamycin, linezolid, teicoplanin, meropenem, cefazolin, and daptomycin. AKI was diagnosed according to the KDIGO serum creatinine (SCr) criteria. Cumulative incidence curves were estimated using the Aalen-Johansen method and adjusted for confounding and selection bias through inverse probability of treatment and censoring weighting. Given the time lag of 24-48 h between changes in renal function and SCr, we summarized the estimates by calculating the absolute risks and risk differences at both 2 and 14 days after initiation.
Results: We included 1809 ICU admissions. After adjustment, vancomycin was associated with a higher risk of AKI at 14 days of follow-up compared to the alternative antibiotics (0.28 [95% confidence interval (CI) 0.21-0.34] vs. 0.17 [95% CI 0.14-0.20]; risk difference 0.11 [95% CI 0.04-0.19]), but not at 2 days of follow-up (0.10 [95% CI 0.06-0.12] vs. 0.10 [95% CI 0.08-0.11]; risk difference 0.00 [95% CI -0.03-0.03]).
Conclusions: Our findings indicate that vancomycin causes a higher risk of AKI compared to the alternative antibiotics. We recommend clinicians to be compliant with vancomycin-induced AKI prevention strategies, such as therapeutic drug monitoring or the consideration of an alternative antibiotic if possible.
Keywords: acute kidney injury; adverse drug event; intensive care; target trial emulation; vancomycin.
© 2025 The Author(s). Pharmacoepidemiology and Drug Safety published by John Wiley & Sons Ltd.
Conflict of interest statement
For the duration of this research, Giovanni Cinà has held a partial appointment at the company Pacmed, as well as stock appreciation options. The other authors declare no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

