Pollen sterols are highly diverse but phylogenetically conserved
- PMID: 40878762
- PMCID: PMC12445805
- DOI: 10.1111/nph.70470
Pollen sterols are highly diverse but phylogenetically conserved
Abstract
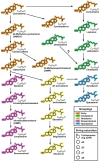
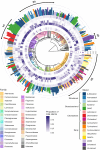
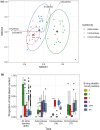
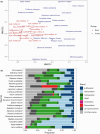
Phytosterols regulate cell membrane fluidity and are precursors for plant hormones and secondary metabolites in plants. Insects are auxotrophic for sterols; therefore, they have to consume phytosterols and dealkylate them to cholesterol. Some insects, including bees, which rely on dietary sources, primarily pollen, for sterols, cannot modify them; therefore, they have adapted to use them unmodified as they occur in pollen. Here, using high resolution chemical analysis, we describe the distribution of 78 different pollen sterols from 295 UK wildflower taxa and use this data to develop an evolutionary rationale for the diversity of sterols in pollen compared to vegetative tissues. The sterol occurrence in pollen was a function of plant lineage and conserved in groups as high as subfamily. Most pollen in the present study contained high proportions of Δ5 sterols including β-sitosterol, 24-methylenecholesterol and isofucosterol, which are important sterols for bees. The sterols recorded in honeybees occurred in the pollen of only 68% of plant taxa; however, none matched these proportions exactly, suggesting they must forage pollen from multiple plant taxa to satisfy their sterol requirements. We conclude that there is evidence for pollen sterol composition being the result of diverse driving forces including plant lineage and potentially pollinator nutritional requirements.
Keywords: phylogeny; phytosterol; plant lipids; pollen nutrients; pollinator guild.
© 2025 The Author(s). New Phytologist © 2025 New Phytologist Foundation.
Conflict of interest statement
None declared.
Figures


References
-
- Bakker JD. 2024. Applied multivariate statistics in R . University of Washington. [WWW document] URL https://uw.pressbooks.pub/appliedmultivariatestatistics/ [accessed 11 August 2025].
-
- Behmer ST. 2017. Overturning dogma: tolerance of insects to mixed‐sterol diets is not universal. Current Opinion in Insect Science 23: 89–95. - PubMed
-
- Behmer ST, Elias DO. 2000. Sterol metabolic constraints as a factor controbuting to the maintenance of diet mixing in grasshoppers (Orthoptera: Acrididae). Physiological and Biochemical Zoology 73: 219–230. - PubMed
-
- Behmer ST, Nes WD. 2003. Insect sterol nutrition and physiology: a global overview. In: Simpson S, ed. Advances in insect physiology, vol. 31. London, UK: Academic Press, 1–72.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

