Real World Effectiveness and Tolerability of Novel Monoclonal Antibodies and Rituximab for NMOSD
- PMID: 40878863
- PMCID: PMC12516216
- DOI: 10.1002/acn3.70160
Real World Effectiveness and Tolerability of Novel Monoclonal Antibodies and Rituximab for NMOSD
Abstract
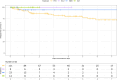
In this multicenter retrospective cohort study of 135 people with aquaporin-4 IgG+ neuromyelitis optica spectrum disorder (NMOSD), some of whom were exposed to multiple therapies, we evaluated the effectiveness and tolerability of rituximab (n = 111) and novel monoclonal antibodies (nMAbs): eculizumab (n = 9), inebilizumab (n = 23), and satralizumab (n = 14). Over a median follow-up of 3.92 years, 21/111 rituximab-treated patients relapsed. In contrast, relapse occurred in only 1/23 inebilizumab-treated patients (median follow-up 1.27 years), with no relapses observed in those receiving eculizumab or satralizumab. Twelve-month relapse-free probabilities were 92% (rituximab), 94% (inebilizumab), and 100% (eculizumab and satralizumab). Among those with available MRI data, 10/73 rituximab and 1/14 inebilizumab developed new lesions. There were no serious adverse events. These findings support nMAbs as effective and well-tolerated first-line therapies for NMOSD.
Keywords: NMOSD; effectiveness; monoclonal antibodies; rituximab; tolerability.
© 2025 The Author(s). Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Z.J./M.D.: No disclosures, M.R.: received research funding (Roche, Novartis, Biogen, Genentech, Connor B Judge (CBJ) Foundation and National Multiple Sclerosis), patient education funds (Genzyme, CBJ Foundation) also served on the Data Safety Monitoring Committee (DSMC) for Biogen, speaker or consultant for (Serono, Novartis, Genentech, Genzyme, Horizon, TG Therapeutics, Bristol Myers Squibb [BMS]), Cycle and Sanofi and is Founder of Brain Fresh LLC and Co‐Founder of Brain Ops Group. L.H.H. serves/ed as a consultant to Genentech, Novartis, EMD Serono, Genzyme, TG Therapeutics, Horizon, and Alexion; on scientific advisory boards for Novartis; and has received non‐promotional speaker honoraria for TG Therapeutics. She has received research funding paid directly to her institution from Genentech. C.M.H. received speaker, consulting, and/or advisory board fees from Biogen, Novartis, Bristol‐Myers Squibb, EMD Serono, Genentech, Genzyme, TG Therapeutics, Alexion Pharmaceuticals, and Horizon Therapeutics; she also receives research funding paid directly to her institution from Biogen, Novartis, Bristol Myers Squibb, NIH‐NINDS 1U01NS111678‐01A1 sub‐award, and Patient Centered Outcomes Research Institute. J.R.A.: served on scientific advisory boards for EMD Serono, Genentech, Horizon Therapeutics, and TG Therapeutics; has received research support from Horizon Therapeutics. D.S.C.: received grants/contracts from Bristol Myers Squibb, Biogen, Novartis, EMD Serono, Amgen. Received consulting fees from Biogen, Novartis, TG Therapeutics, and Genentech. D.O.: received research support from the National Institutes of Health, National Multiple Sclerosis Society, Patient Centered Outcomes Research Institute, Race to Erase MS Foundation, Agency for Healthcare Research and Quality, Genentech, Genzyme, and Novartis. Received consulting fees from Biogen Idec, Bristol Myers Squibb, Genentech/Roche, Genzyme, Novartis, Merck, Sanofi, and Pipeline Therapeutics. J.A.C.: received personal compensation for consulting for Astoria, Bristol‐Myers Squibb, Convelo, and Viatris, and chairing a DSMB for Celltrion. A.K.: received compensation from Genentech, Alexion, and Horizon Therapeutics.
Figures

References
-
- Traboulsee A., Greenberg B. M., Bennett J. L., et al., “Safety and Efficacy of Satralizumab Monotherapy in Neuromyelitis Optica Spectrum Disorder: A Randomised, Double‐Blind, Multicentre, Placebo‐Controlled Phase 3 Trial,” Lancet Neurology 19, no. 5 (2020): 402–412, 10.1016/S1474-4422(20)30078-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

