Clinical Validation of Loop-Mediated Isothermal Amplification Assays for the Rapid Detection of Neisseria gonorrhoeae and Chlamydia trachomatis
- PMID: 40879309
- PMCID: PMC12395580
- DOI: 10.1111/1751-7915.70214
Clinical Validation of Loop-Mediated Isothermal Amplification Assays for the Rapid Detection of Neisseria gonorrhoeae and Chlamydia trachomatis
Abstract
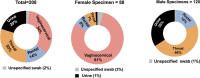
Neisseria gonorrhoeae (GC) and Chlamydia trachomatis (CT) are the predominant causes of bacterial sexually transmitted diseases. While nucleic acid amplification testing (NAATs), primarily polymerase chain reaction (PCR), is regarded as the gold standard for identifying these two pathogens, it usually takes a prolonged turnaround time and requires sophisticated equipment with considerable expense. In this study, we developed novel loop-mediated isothermal amplification (LAMP) assays for rapid detection (< 30 min) of GC and CT in clinical urine and swab specimens. We analysed 208 clinical samples with three different pre-treatment techniques including heating inactivation, centrifugation, and DNA extraction. LAMP results were compared with clinical results from the FDA-approved BD ProbeTec ET assay. After heating inactivation, LAMP detected merely 41% and 65% of BD-identified GC- and CT-positive samples, respectively. Introducing centrifugation as an affordable and rapid pre-treatment step increased detection rates to 81% and 91% for GC and CT, respectively. DNA extraction further enhanced the detection rates to 96% and 95% for GC- and CT-LAMP, respectively. All these LAMP assays exhibited clinical specificity of ≥ 98%, underscoring the specificity of the chosen target genes (the porA pesudogene for GC and the ftsK gene for CT). Discrepant samples were verified by real-time PCR; results were consistent with our LAMP findings. The overall LAMP performance met the WHO criteria for sensitivity and specificity for GC/CT point-of-care testing.
Keywords: Chlamydia trachomatis; Neisseria gonorrhoeae; LAMP assay; Sexually Transmitted Infectious; diagnosis; point‐of‐care testing (POCT).
© 2025 The Author(s). Microbial Biotechnology published by John Wiley & Sons Ltd.
Conflict of interest statement
This project is academic research funded by Prenetics Inc., Hong Kong, China. Patents have been filed by Oxford University Innovation based on this manuscript.
Figures



Similar articles
-
Systematic review: noninvasive testing for Chlamydia trachomatis and Neisseria gonorrhoeae.Ann Intern Med. 2005 Jun 7;142(11):914-25. doi: 10.7326/0003-4819-142-11-200506070-00010. Ann Intern Med. 2005. PMID: 15941699
-
Rapid Identification of Neisseria gonorrhoeae, Chlamydia trachomatis, and Ureaplasma urealyticum by RNA Amplification Lateral Flow Assay.J Vis Exp. 2025 Jun 20;(220). doi: 10.3791/68413. J Vis Exp. 2025. PMID: 40622877
-
Detection of Neisseria gonorrhoeae and Chlamydia trachomatis by PCR in a sample pool (urine, rectum and pharynx) in asymptomatic patients at risk of sexually transmitted infections.Enferm Infecc Microbiol Clin (Engl Ed). 2025 Aug-Sep;43(7):374-377. doi: 10.1016/j.eimce.2024.11.009. Enferm Infecc Microbiol Clin (Engl Ed). 2025. PMID: 40754349
-
Application of Loop-Mediated Isothermal Amplification Assay for the Detection of Chlamydia trachomatis and Neisseria gonorrhoeae.Methods Mol Biol. 2019;2042:19-25. doi: 10.1007/978-1-4939-9694-0_3. Methods Mol Biol. 2019. PMID: 31385267
-
Home-based versus clinic-based specimen collection in the management of Chlamydia trachomatis and Neisseria gonorrhoeae infections.Cochrane Database Syst Rev. 2015 Sep 29;2015(9):CD011317. doi: 10.1002/14651858.CD011317.pub2. Cochrane Database Syst Rev. 2015. PMID: 26418128 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

