PTP1B deficiency in myeloid cells increases susceptibility to Candida albicans systemic infection by modulating antifungal immunity
- PMID: 40879376
- PMCID: PMC12505889
- DOI: 10.1128/mbio.01516-25
PTP1B deficiency in myeloid cells increases susceptibility to Candida albicans systemic infection by modulating antifungal immunity
Abstract
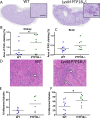
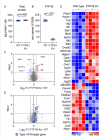
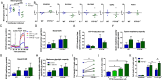
Invasive candidiasis, primarily caused by Candida albicans, poses a significant threat to immunocompromised patients, with high mortality rates. Understanding how immune responses to Candida albicans are mounted and controlled is fundamental to developing new therapeutic strategies. Protein tyrosine phosphatase 1B (PTP1B) is a regulator of immunoreceptor signaling and downstream inflammatory and metabolic responses and a pharmaceutical target. Here, we reveal a critical role for myeloid cell-intrinsic PTP1B in antifungal immunity. Mice lacking PTP1B in myeloid cells (LysM PTP1B-/-) were significantly more susceptible to systemic C. albicans infection, exhibiting lower survival, greater weight loss, and elevated fungal burdens in the kidney, liver, and brain. These mice also showed heightened proinflammatory mRNA expression in organs and increased kidney tubular inflammation, with increased leukocyte infiltration and chemokine production, contributing to immunopathology. Neutrophils from LysM PTP1B-/- mice displayed impaired maturation in infected kidneys and reduced reactive oxygen species production in vitro. Proteomic profiling of infected bone marrow-derived macrophages (BMDMs) revealed significant enrichment of type I interferon-regulated proteins in the absence of PTP1B. These BMDMs showed impaired phagocytosis, reduced killing capacity, and lower viability during infection, phenotypes recapitulated in human macrophages treated with a pharmacological PTP1B inhibitor. Collectively, our findings highlight PTP1B as a key modulator of innate immune responses to C. albicans, balancing antifungal activity and systemic toxicity with inflammation and metabolic fitness. Boosting specific PTP1B-dependent pathways may offer new strategies for enhancing host defense while minimizing fungal-induced immunopathology.IMPORTANCESystemic Candida albicans infections are a leading cause of hospital-acquired morbidity and mortality, particularly in immunocompromised individuals and patients receiving immunosuppressive treatments. Despite antifungal therapies, outcomes remain poor, underscoring the need to better understand host factors that control fungal clearance. Protein tyrosine phosphatase 1B (PTP1B) is a key intracellular regulator of immune and metabolic signaling. This study identifies a critical role for myeloid PTP1B in antifungal defense and susceptibility to systemic C. albicans infection. Loss of PTP1B impairs neutrophil and macrophage function, disrupts inflammatory balance, and compromises pathogen clearance. These findings reveal PTP1B as a central modulator of immune responses to C. albicans and highlight its potential as a target for host-directed therapies to improve outcomes in systemic fungal infections.
Keywords: Candida albicans; infections; macrophages; protein tyrosine phosphatase 1B.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
