Evaluation of methods for the measurement of antibody-dependent enhancement of dengue virus infection using different FcγRIIa expressing cell lines
- PMID: 40880375
- PMCID: PMC12396659
- DOI: 10.1371/journal.pone.0331320
Evaluation of methods for the measurement of antibody-dependent enhancement of dengue virus infection using different FcγRIIa expressing cell lines
Abstract
Background: Pre-existing dengue antibodies could potentially exacerbate disease severity through antibody-dependent enhancement (ADE). Current serological assays focus on measuring neutralizing antibodies for vaccine evaluation, but don't measure sub-neutralizing antibodies that enhance infection via Fcγ receptors. Consensus on a standardized system for measuring dengue virus ADE remains elusive.
Methods: In this study, we compared and evaluated ADE responses using two different methodologies in healthy blood donors (n = 12) and secondary dengue patients' (n = 12) samples with pre-existing IgG antibodies to dengue virus (DENV). We performed an ADE-infection assay in FcγRIIa-expressing U937, K562, and Vero-CD32a cells. Foci-reduction neutralization test (FRNT) was performed simultaneously in Vero and Vero-CD32a cells, and reduction in neutralization titres was examined in Vero-CD32a cells.
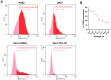
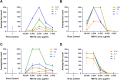
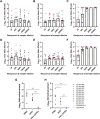
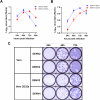
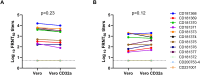
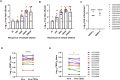
Results: Out of 12 blood donors, all 9 anti-dengue IgG-positive donors demonstrated ADE through infection-enhancement assay against DENV-2 and DENV-4 serotypes in U937 and K562 cells, but not in Vero-CD32a cells. None of the anti-dengue IgG-negative donor samples exhibited ADE against DENV in all three cell lines. Fold-enhancement of DENV-2 infection was comparable in the two cell lines whereas, fold-enhancement of DENV-4 infection was significantly higher in K562 than in U937 cells. Comparable neutralizing antibody titres in Vero and Vero-CD32a cells against DENV-2 and DENV-4 serotypes suggest that donor samples did not exhibit any enhancing activity in Vero-CD32a cells. Comparable DENV-2 titres and significantly lower DENV-4 titres were obtained in Vero-CD32a than in Vero cells in secondary dengue patient samples, indicating that enhancing activity was influenced by DENV serotypes.
Conclusion: In summary, infection-enhancement assay using K562 cells was superior to U937 and Vero-CD32a cells in evaluating ADE. Samples with high neutralizing activity demonstrated very low levels of infection-enhancing activity in Vero-CD32a cells. Comparison of FRNT titres in Vero and Vero-CD32a cells is not suitable for detecting ADE. Our findings suggest that infection-enhancing activities are apparent at sub-neutralizing concentrations of dengue virus antibodies in all individuals exposed to dengue virus.
Copyright: © 2025 Chelluboina et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Rodrigo WWSI, Jin X, Blackley SD, Rose RC, Schlesinger JJ. Differential enhancement of dengue virus immune complex infectivity mediated by signaling-competent and signaling-incompetent human Fcgamma RIA (CD64) or FcgammaRIIA (CD32). J Virol. 2006;80(20):10128–38. doi: 10.1128/JVI.00792-06 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

