Nitrogen status exerts dynamic control over phosphorus sensing and acquisition via PSR1 in colimited marine diatoms
- PMID: 40880470
- PMCID: PMC12396336
- DOI: 10.1126/sciadv.adw8260
Nitrogen status exerts dynamic control over phosphorus sensing and acquisition via PSR1 in colimited marine diatoms
Abstract
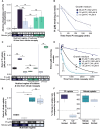
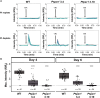
Nutrient availability controls phytoplankton growth in aquatic ecosystems globally. Phytoplankton frequently experience a limiting supply of multiple nutrients simultaneously (colimitation). Ocean warming is predicted to exacerbate marine nutrient limitation. Yet, how phytoplankton adapt their physiology and regulate responses to colimitation is poorly understood. Here, we show that when the crucial macronutrients nitrogen (N) and phosphorus (P) colimit growth of globally abundant phytoplankton, the diatoms, cellular resources are diverted to prioritize N uptake over P sensing and acquisition. Regulatory mechanisms for responding to fluctuating P supply, including Phosphate Starvation Response 1 (PSR1) and P-resupply sensing via Ca2+ signaling, are strictly nitrate dependent. Further, P-Ca2+ signaling is impaired in psr1 mutants, suggesting that PSR1 is necessary for this response and coordinates adaptations to colimitation. Our study demonstrates a hierarchy of resource allocation during colimitation that points to N starvation mechanisms overriding those of P and could lead to N-P colimitation being masked in many marine ecosystems.
Figures





Similar articles
-
Nutrient colimitation is a quantitative, dynamic property of microbial populations.Proc Natl Acad Sci U S A. 2024 Dec 24;121(52):e2400304121. doi: 10.1073/pnas.2400304121. Epub 2024 Dec 18. Proc Natl Acad Sci U S A. 2024. PMID: 39693349 Free PMC article.
-
A Novel Ca2+ Signaling Pathway Coordinates Environmental Phosphorus Sensing and Nitrogen Metabolism in Marine Diatoms.Curr Biol. 2021 Mar 8;31(5):978-989.e4. doi: 10.1016/j.cub.2020.11.073. Epub 2020 Dec 28. Curr Biol. 2021. PMID: 33373640
-
Chlorophyll trends are negative for lakes but positive for estuarine-coastal waters.Proc Natl Acad Sci U S A. 2025 Jul 15;122(28):e2502289122. doi: 10.1073/pnas.2502289122. Epub 2025 Jul 7. Proc Natl Acad Sci U S A. 2025. PMID: 40623175
-
Mineral nutrient acquisition under stress: Sensing, signaling, and transportation.Plant Sci. 2025 Aug 6;360:112702. doi: 10.1016/j.plantsci.2025.112702. Online ahead of print. Plant Sci. 2025. PMID: 40780576 Review.
-
Phosphorus physiological ecology and molecular mechanisms in marine phytoplankton.J Phycol. 2016 Feb;52(1):10-36. doi: 10.1111/jpy.12365. Epub 2016 Jan 11. J Phycol. 2016. PMID: 26987085 Review.
References
-
- Moore C. M., Mills M. M., Arrigo K. R., Berman-Frank I., Bopp L., Boyd P. W., Galbraith E. D., Geider R. J., Guieu C., Jaccard S. L., Jickells T. D., La Roche J., Lenton T. M., Mahowald N. M., Marañón E., Marinov I., Moore J. K., Nakatsuka T., Oschlies A., Saito M. A., Thingstad T. F., Tsuda A., Ulloa O., Processes and patterns of oceanic nutrient limitation. Nat. Geosci. 6, 701–710 (2013).
-
- Wu J., Sunda W., Boyle E. A., Karl D. M., Phosphate depletion in the Western North Atlantic Ocean. Science 289, 759–762 (2000). - PubMed
-
- Thingstad T. F., Krom M. D., Mantoura R. F. C., Flaten G. A. F., Groom S., Herut B., Kress N., Law C. S., Pasternak A., Pitta P., Psarra S., Rassoulzadegan F., Tanaka T., Tselepides A., Wassmann P., Woodward E. M. S., Riser C. W., Zodiatis G., Zohary T., Nature of phosphorus limitation in the ultraoligotrophic Eastern Mediterranean. Science 309, 1068–1071 (2005). - PubMed
-
- Ly J., Philippart C. J. M., Kromkamp J. C., Phosphorus limitation during a phytoplankton spring bloom in the western Dutch Wadden Sea. J. Sea Res. 88, 109–120 (2014).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

