Cytoskeletal control in adult microglia is essential to restore neurodevelopmental synaptic and cognitive deficits
- PMID: 40880479
- PMCID: PMC12396340
- DOI: 10.1126/sciadv.adw0128
Cytoskeletal control in adult microglia is essential to restore neurodevelopmental synaptic and cognitive deficits
Abstract
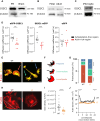
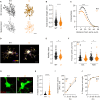
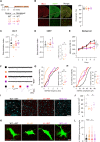
Synaptic dysfunction is a hallmark of neurodevelopmental disorders (NDDs), often linked to genes involved in cytoskeletal regulation. While the role of these genes has been extensively studied in neurons, microglial functions such as phagocytosis are also dependent on cytoskeletal dynamics. We demonstrate that disturbance of actin cytoskeletal regulation in microglia, modeled by genetically impairing the scaffold protein Disrupted-in-Schizophrenia 1 (DISC1), which integrates actin-binding proteins, causes a shift in actin regulatory balance favoring filopodial versus lamellipodial actin organization. The resulting microglia-specific dysregulation of actin dynamics leads to excessive uptake of synaptic proteins. Genetically engineered DISC1-deficient mice show diminished hippocampal excitatory transmission and associated spatial memory deficits. Reintroducing wild-type microglia-like cells via bone marrow transplantation in adult DISC1-deficient mice restores the synaptic function of neurons and rescues cognitive performance. These findings reveal a pivotal role for microglial actin cytoskeletal remodeling in preserving synaptic integrity and cognitive health. Targeting microglial cytoskeletal dynamics may effectively address cognitive impairments associated with NDDs, even in adulthood.
Figures


References
-
- Salter M. W., Stevens B., Microglia emerge as central players in brain disease. Nat. Med. 23, 1018–1027 (2017). - PubMed
-
- Reisinger S., Khan D., Kong E., Berger A., Pollak A., Pollak D. D., The poly(I:C)-induced maternal immune activation model in preclinical neuropsychiatric drug discovery. Pharmacol. Ther. 149, 213–226 (2015). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

