Activation of WNK1 signaling through Piezo1
- PMID: 40880539
- PMCID: PMC12415193
- DOI: 10.1073/pnas.2513155122
Activation of WNK1 signaling through Piezo1
Abstract
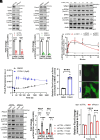
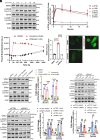
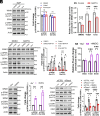
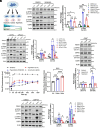
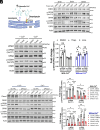
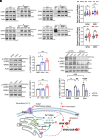
With No lysine (K) 1 (WNK1) is essential for ion and volume homeostasis, sensing osmotic stress and activating pathways that regulate ion transport. Its response to osmotic stress shares similarities with the function of the mechanosensitive ion channel Piezo1. In this study, we show that Yoda1, a Piezo1 agonist, activates WNK1 downstream kinase targets oxidative stress-responsive 1 (OSR1) and STE20/SPS1-related serine, proline-, and alanine-rich kinase (SPAK) in endothelial cells within minutes. Ionophore-induced Ca2+ influx similarly triggers their activation. Comparable responses were observed in HDMEC, HUVEC, A549, MDA-MB-231, and HeLa cells. Hypotonic stress also enhances SPAK and OSR1 phosphorylation, which is attenuated by WNK1 inhibition or Piezo1 knockdown, whereas hypertonic stress-induced phosphorylation is not affected by Piezo1 knockdown. Chelating Ca2+ or depleting intracellular stores prevents their activation, while increasing intracellular Ca2+ via the Na+/Ca2+ exchanger or thapsigargin enhances it. ER-released Ca2+ is sufficient to activate SPAK and OSR1 even in the absence of extracellular Ca2+, and this effect is diminished by Piezo1 knockdown. Both Yoda1 and ionomycin promote phosphorylation of WNK1 at serine 382, a modification that increases its catalytic activity. These findings identify Piezo1 as an activator of WNK1, linking Ca2+ dynamics to WNK1-OSR1/SPAK signaling.
Keywords: OSR1; Piezo1; SPAK; WNK1; osmotic stress.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures
References
-
- Moriguchi T., et al. , WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J. Biol. Chem. 280, 42685–42693 (2005). - PubMed
-
- Wilson F. H., et al. , Human hypertension caused by mutations in WNK kinases. Science 293, 1107–1112 (2001). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

