Advances in nanomechanical property mapping by atomic force microscopy
- PMID: 40880595
- PMCID: PMC12379807
- DOI: 10.1039/d5na00702j
Advances in nanomechanical property mapping by atomic force microscopy
Abstract
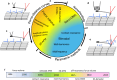
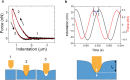
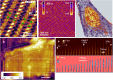
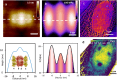
AFM-based mechanical property measurements are widely used in energy storage, polymer science, mechanobiology or nanomedicine. Mechanical properties are determined by expressing the experimental force in terms of a contact mechanics model. A nanomechanical map is generated by representing one or more mechanical parameters as a function of the tip's spatial coordinates. Force spectroscopy modes might be separated into two categories, adhesion and indentation. Here we describe the principles of AFM-based indentation modes to generate spatially resolved maps of the mechanical properties at the nanoscale. The review provides an update on the progress in nanomechanical mapping since 2019. The focus is on quantitative accuracy, spatial resolution, high-speed data acquisition, machine learning and viscoelastic property mapping. Two advanced applications which emerged from AFM-based indentation modes, nanomechanical tomography and volume imaging of solid-liquid interfaces, are also described.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Figures






Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Anterior Approach Total Ankle Arthroplasty with Patient-Specific Cut Guides.JBJS Essent Surg Tech. 2025 Aug 15;15(3):e23.00027. doi: 10.2106/JBJS.ST.23.00027. eCollection 2025 Jul-Sep. JBJS Essent Surg Tech. 2025. PMID: 40821726 Free PMC article.
-
Mapping heterogeneity of cellular mechanics by multi-harmonic atomic force microscopy.Nat Protoc. 2018 Oct;13(10):2200-2216. doi: 10.1038/s41596-018-0031-8. Nat Protoc. 2018. PMID: 30218102
-
123I-MIBG scintigraphy and 18F-FDG-PET imaging for diagnosing neuroblastoma.Cochrane Database Syst Rev. 2015 Sep 29;2015(9):CD009263. doi: 10.1002/14651858.CD009263.pub2. Cochrane Database Syst Rev. 2015. PMID: 26417712 Free PMC article.
-
Factors that impact on the use of mechanical ventilation weaning protocols in critically ill adults and children: a qualitative evidence-synthesis.Cochrane Database Syst Rev. 2016 Oct 4;10(10):CD011812. doi: 10.1002/14651858.CD011812.pub2. Cochrane Database Syst Rev. 2016. PMID: 27699783 Free PMC article.
References
-
- Binnig G. Quate C. F. Gerber C. Atomic Force Microscope. Phys. Rev. Lett. 1986;56:930–933. - PubMed
-
- Garcia R., Amplitude Modulation AFM, Wiley-VCH, 2011
-
- Heinz W. F. Hoh J. H. Spatially resolved force spectroscopy of biological surfaces using the atomic force microscope. Trends Biotechnol. 1999;17:143–150. - PubMed
-
- Garcia R. Proksch R. Nanomechanical mapping of soft matter by bimodal force microscopy. Eur. Polym. J. 2013;49:1897–1906.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

