Controlling Molecular Packing in Aqueous Metallosupramolecular Self-assembly by Ligand Geometry
- PMID: 40880891
- PMCID: PMC12382264
- DOI: 10.1021/prechem.3c00058
Controlling Molecular Packing in Aqueous Metallosupramolecular Self-assembly by Ligand Geometry
Abstract
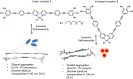
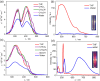
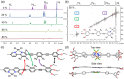
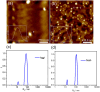
The coordination geometry of d8 transition metal complexes has been successfully exploited as a tool to tune photophysical properties and self-assembly pathways of supramolecular polymerization processes, with a focus being primarily placed on organic media. Expanding such controlled supramolecular and photophysical properties to assemblies in aqueous media by molecular design is, however, still challenging due to the difficulty in programming noncovalent interactions in water. Herein, we tackle this challenge by analyzing the aqueous self-assembly of amphiphilic Pt-(II) complexes of different molecular geometry in order to control self-assembly and metal-metal interactions in aqueous media. To this end, we have designed two Pt-(II) complexes, 1 and 2, containing an identical oligophenyleneethynylene (OPE)-based aromatic scaffold that differ in the molecular geometry (linear vs V-shaped) imposed by ligand substitution and studied their comparative self-assembly behavior in aqueous media. Even though both molecules follow the isodesmic mechanism of self-assembly, their structural difference strongly influences the molecular packing in aqueous media, which in turn impacts the photophysical properties (i.e. absence or presence of MMLCT) and the self-assembly outcome. While the molecular geometry for 2 enforces short Pt···Pt contacts driven by an efficient face-to-face stacking of the OPE backbone, the antiparallel packing of 1 with slight translational offset does not allow the formation of short Pt···Pt contacts. Such a distinct interplay of interactions for 1 and 2 in aqueous media leads to significant differences in photoluminescence.
Keywords: Pt(II) complexes; Self-assembly; amphiphilic systems; supramolecular polymerization; π-conjugated systems.
© 2023 The Authors. Co-published by University of Science and Technology of China and American Chemical Society.
Figures


References
-
- Hashim P. K., Bergueiro J., Meijer E. W., Aida T.. Supramolecular Polymerization: A Conceptual Expansion for Innovative Materials. Prog. Polym. Sci. 2020;105:101250. doi: 10.1016/j.progpolymsci.2020.101250. - DOI
-
- MacFarlane L. R., Shaikh H., Garcia-Hernandez J. D., Vespa M., Fukui T., Manners I.. Functional nanoparticles through π-conjugated polymer self-assembly. Nat. Rev. Mater. 2021;6:7–26. doi: 10.1038/s41578-020-00233-4. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
