Fasting elicits gut microbiome signature changes that extend to type 1 diabetes patients
- PMID: 40881123
- PMCID: PMC12380539
- DOI: 10.3389/fendo.2025.1623800
Fasting elicits gut microbiome signature changes that extend to type 1 diabetes patients
Abstract
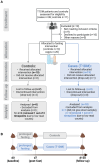
The gut microbiome has been linked to the pathogenesis of type 1 diabetes (T1D), identifying it as a promising therapeutic target. Nutritional interventions, which are an effective way to modulate the gut microbiome, thus show potential to be applied as complementary therapies for T1D. One particular dietary intervention, prolonged therapeutic fasting, has been shown to ameliorate symptoms of several autoimmune diseases, while also modifying the gut microbiota composition of healthy populations. It is unclear, however, how the gut microbiota of patients suffering from diseases of autoimmunity will respond to fasting. In this pilot study, we investigate the effects of prolonged fasting on the gut microbiome of T1D patients: Fasting substantially changed the composition and structure of the T1D gut microbiome so that it converged with that of non-diabetic controls immediately post fasting. Moreover, a comparison with a population of patients suffering from Multiple Sclerosis revealed substantial overlap in post-fasted microbiome changes and a remarkable consistency with published data of non-autoimmune populations, indicating that fasting leads to signature microbiome changes that are independent of host health status and disease type. A correlation analysis between fasting-mediated microbiota modifications and changes in clinical parameters revealed several significant associations between the Oscillospiraceae and Lachnospiraceae families and cholesterol and blood pressure changes in the T1D cohort, corroborating previous studies reporting on these associations in non-diabetic subjects. In conclusion, the observed fasting-mediated microbiome signature suggests that nutrient availability is a major disease-independent factor in shaping gut microbiome composition, likely driven by the need for metabolic diversification of microbial nutrient acquisition. The corresponding clinical associations highlight the need to investigate if these fasting-driven changes in the reported taxa are causally linked to the recorded clinical benefits of therapeutic fasting and what importance fasting as an additional therapeutic intervention might have to improve long term conditions in people with T1D.
Keywords: autoimmunity; fasting; gut bacteria; gut microbiome; multiple sclerosis; nutrient availability; therapeutic fasting; type 1 diabetes.
Copyright © 2025 Graef, Berger, Bahr, Stange, Michalsen, Paul, Vallance and Jacobson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures




References
-
- Lerner A, Jeremias P, Matthias T. The world incidence and prevalence of autoimmune diseases is increasing. Int J Celiac Dis. (2016) 3:151–5. doi: 10.12691/ijcd-3-4-8 - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

