Ellagic acid on milk production performance, blood and milk hormones, antioxidant capacity and fecal microbial communities in lactating Yili mares
- PMID: 40881293
- PMCID: PMC12380849
- DOI: 10.3389/fmicb.2025.1656100
Ellagic acid on milk production performance, blood and milk hormones, antioxidant capacity and fecal microbial communities in lactating Yili mares
Abstract
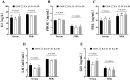
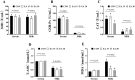
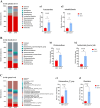
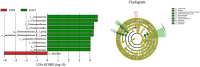
Ellagic acid (EA), a natural polyphenol, exerts potent antioxidant and anti-inflammatory effects in humans and other animals, while contributing to intestinal microbiota homeostasis. This study investigated the impact of EA supplementation on milk production, hormone secretion, antioxidant activity, and gut microbiota in lactating Yili mares. Eighteen lactating Yili mares with an average body weight of 400.06 ± 15.01 kg, average age of 9.89 ± 0.83 years, with similar parity (5-6 foalings) were used in this study. These mares had foaled in May (first foal born on May 7, last foal born on May 13) and had been lactating for 30 days at the initiation of the experiment. They were randomly allocated to 3 groups (n = 6 per group): a control group (CON) receiving no EA supplementation, the EA15 group (15 mg/kg BW/day EA), and the EA30 group (30 mg/kg BW/day EA). The supplementation trial commenced on lactation day 30 (study day 0) and continued for 90 days. By study days 60 and 90, EA supplementation enhanced milk production in lactating mares. On study day 30, serum prolactin (PRL) concentrations were increased in mares in the EA15 group, and milk PRL concentrations were increased in mares in the EA30 group compared to the CON group of mares. Conversely, serum luteinizing hormone (LH) concentrations and milk growth hormone (GH) concentrations were reduced. Compared to the CON group of mares, mares in the EA15 group had increased serum glutathione peroxidase (GSH-Px) activity, and mares in the EA30 group had increased milk superoxide dismutase (SOD) and catalase (CAT) activities, while reducing milk total antioxidant capacity (T-AOC) and malondialdehyde (MDA) levels. Supplementation with EA increased the relative abundance of Actinobacteriota, Verrucomicrobiota, Christensenellaceae, Coriobacteriales_Incertae_Sedis, Christensenellaceae_R_7_group, and Phoenicibacter in the feces of lactating mares, while decreasing the relative abundance of Proteobacteria, Moraxellaceae, and Acinetobacter. Overall, EA supplementation increases milk production in lactating Yili mares, modulates lactation-associated hormone secretion, improves the body's antioxidant capacity, and alters the composition of the intestinal microflora. The results suggest potential applications of EA supplementation in equine nutrition strategies aimed at improving lactation performance and antioxidant status during lactation. Future research could focus on optimizing dosage regimens and validating its efficacy in larger-scale production systems to facilitate practical application in equine husbandry.
Keywords: Yili mares; antioxidant; ellagic acid; fecal microorganism; hormones; lactation performance.
Copyright © 2025 Huang, He, Ma, Li, Li, Zang, Hou and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Effects of increasing doses of red yeast rice polyphenol extract on apparent digestibility, antioxidant activity, milk production, and fatty acid in lactating dairy goats.J Anim Sci. 2025 Jan 4;103:skaf167. doi: 10.1093/jas/skaf167. J Anim Sci. 2025. PMID: 40360291
-
Exploring optimal folic acid supplementation levels for lactating-pregnant rabbit does with different litter size.J Anim Sci. 2024 Jan 3;102:skae340. doi: 10.1093/jas/skae340. J Anim Sci. 2024. PMID: 39503276
-
Changes of fecal microbiota with supplementation of Acremonium terricola culture and yeast culture in ewes during lactation.J Anim Sci. 2025 Jan 4;103:skaf174. doi: 10.1093/jas/skaf174. J Anim Sci. 2025. PMID: 40380819
-
Effects of rumen-protected choline supplementation on lactation performance of dairy cows: A systematic review and dose-response meta-analysis.J Dairy Sci. 2025 Sep;108(9):9516-9534. doi: 10.3168/jds.2024-25844. Epub 2025 Jul 3. J Dairy Sci. 2025. PMID: 40614817
-
Effects of sevoflurane versus other general anaesthesia on emergence agitation in children.Cochrane Database Syst Rev. 2014 Sep 12;2014(9):CD007084. doi: 10.1002/14651858.CD007084.pub2. Cochrane Database Syst Rev. 2014. PMID: 25212274 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

