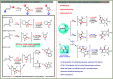
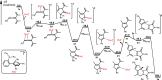
Divergent Synthesis of Polycyclic N‑Heterocycles via Controllable Cyclization of Divinylallenes Catalyzed by Copper and Gold
- PMID: 40881415
- PMCID: PMC12381710
- DOI: 10.1021/jacsau.5c00760
Divergent Synthesis of Polycyclic N‑Heterocycles via Controllable Cyclization of Divinylallenes Catalyzed by Copper and Gold
Abstract
The generation of metal carbenes from readily available allenes represents a remarkable advance in metal carbene chemistry. However, most of these transformations are mainly restricted to the noble-metal catalysts (Au and Pt). Here, a copper-catalyzed desymmetric cyclization reaction of divinylallenes is described, enabling the practical and atom-economical synthesis of a diverse array of valuable triazolo-fused pyridazines and tetracyclic N-heterocycles by a presumable copper carbene intermediate and a highly selective 1,2-N shift process. In contrast to copper catalysis, tricyclic N-heterocycles are formed exclusively under gold catalysis via a presumable 1,2-H-migration. Catalyst-controlled migration is achieved by effective catalyst-controlled site selectivity. In addition, in the presence of oxidant, the copper-catalyzed desymmetric oxidation reaction affords the divergent formation of cyclopentanone-fused tricyclic heterocycles in a one-pot process, where no 1,2-migration into the copper carbenes is observed. Importantly, the mechanistic rationale for this allene cyclization, in particular, accounting for the distinct migration into metal carbenes, is also strongly supported by theoretical calculations.
Keywords: 1,2-N migration; copper; desymmetrization; divinylallenes; donor/donor carbenes.
© 2025 The Authors. Published by American Chemical Society.
Figures
Similar articles
-
Generation of Donor/Donor Copper Carbenes through Copper-Catalyzed Diyne Cyclization: Enantioselective and Divergent Synthesis of Chiral Polycyclic Pyrroles.J Am Chem Soc. 2019 Oct 23;141(42):16961-16970. doi: 10.1021/jacs.9b09303. Epub 2019 Oct 9. J Am Chem Soc. 2019. PMID: 31557018
-
Divergent Synthesis of Kopsia and Structurally Related Monoterpenoid Indole Alkaloids: A Non-biomimetic Strategy.Acc Chem Res. 2025 Sep 2;58(17):2781-2791. doi: 10.1021/acs.accounts.5c00461. Epub 2025 Aug 21. Acc Chem Res. 2025. PMID: 40841185
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Redox Approaches to Carbene Generation in Catalytic Cyclopropanation Reactions.Angew Chem Int Ed Engl. 2024 Jul 15;63(29):e202406218. doi: 10.1002/anie.202406218. Epub 2024 Jun 14. Angew Chem Int Ed Engl. 2024. PMID: 38752878 Free PMC article. Review.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
References
LinkOut - more resources
Full Text Sources