Examination of mycoparasites reveals a new type of host-parasite interface and rearranges the taxonomy of Occultifur and Microsporomyces (Cystobasidiomycetes, Basidiomycota)
- PMID: 40881650
- PMCID: PMC11663426
- DOI: 10.3114/sim.2024.109.07
Examination of mycoparasites reveals a new type of host-parasite interface and rearranges the taxonomy of Occultifur and Microsporomyces (Cystobasidiomycetes, Basidiomycota)
Abstract
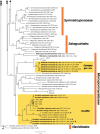
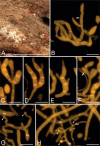
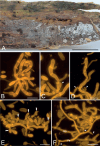
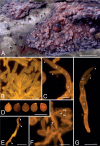
The present study investigates the species boundaries, evolutionary relationships, and host-parasite interfaces of dimorphic mycoparasites that were previously assigned to Achroomyces soranus, Occultifur internus, and Platygloea mycophila based on morphological similarities. Our comparison of recently collected and cultivated samples with the type specimens of A. soranus and P. mycophila shows that both groups are species complexes, of which the taxa can be differentiated based on morphological and ecological characters. By integrating the results of a seven-locus dataset (SSU, LSU, ITS, RPB1, RPB2, TEF1, and mitochondrial CYT-B) and detailed micromorphological comparisons of the investigated specimens, we show for the first time that these three groups of mycoparasites belong to Cystobasidiomycetes (Pucciniomycotina). We applied a polyphasic species concept involving morphology, phylogeny, and ecology to delineate and circumscribe these and new genera. The genus Occultifur comprises six species. Occultifur internus and the newly proposed O. cerinomycicola are intrahymenial mycoparasites producing haustorial cells and establishing fusion pore interaction with their Dacrymycetous host. Based on microscopical examination, we show that Achroomyces soranus is a member of the genus Occultifur. Based on the molecular phylogenetic reconstruction, we found that three lichen-associated fungi which are only known from a yeast morph are nested within Occultifur, i.e. Lichenozyma pisutiana, Microsporomyces cladoniae, and M. wangii. The genus Obvidator is newly introduced for three mycoparasitic species inhabiting members of the corticioid genus Peniophora (Russulales, Agaricomycetes) and causing gall-like malformations of the host basidiome. Microscopic investigation shows that Platygloea mycophila is a member of this genus. Obvidator species display a yet undiscovered type of host-parasite interface, in which the mycoparasites produce short protrusions on their hyphae adjacent to the host hyphae. The lysis of the host cell wall takes place at points of contact with parasite protrusions, but no rupture of the host plasma membrane or nanometer-fusion pore formation was observed. The updated Cystobasidiomycetes phylogeny obtained in this study by including mycoparasites showed that the genera Occultifur and Microsporomyces as currently circumscribed are polyphyletic. To resolve this polyphyly, we introduce two new genera, i.e. Cystastrum and Millanizyma, and recombine species comprising the Occultifur externus clade and a clade consisting of Microsporomyces bloemfonteinensis and M. cladoniophilus, respectively. Taxonomic novelties: New genera: Cystastrum Schoutteten & Yurkov, Millanizyma Schoutteten & Yurkov, Obvidator Schoutteten. New species: Obvidator incarnatae Schoutteten & Yurkov, Obvidator quercinae Schoutteten & Yurkov, Occultifur cerinomycicola Schoutteten, Enzlin & Yurkov. New combinations: Cystastrum brasiliense (F.C.O. Gomes et al.) Schoutteten, Cystastrum externum (J.P. Samp. et al.) Schoutteten & Yurkov, Cystastrum kilbournense (Kurtzman & Robnett ex Denchev & T. Denchev) Schoutteten & Yurkov, Cystastrum mephitis (Zalar et al.) Schoutteten & Yurkov, Cystastrum tropicale (Khunnamw. et al. ex Denchev & T. Denchev) Schoutteten & Yurkov, Cystastrum plantarum (Khunnamw. et al.) Schoutteten & Yurkov, Millanizyma bloemfonteinensis (C.H. Pohl et al.) Schoutteten & Yurkov, Millanizyma cladoniophila (N.H. Nguyen et al.) Schoutteten & Yurkov, Occultifur cladoniae (Kachalkin et al.) Schoutteten, Occultifur pisutianus (Černajová & Škaloud) Schoutteten, Obvidator mycophilus (Burds. & Gilb.) Schoutteten, Occultifur soranus (Hauerslev) Schoutteten, Occultifur wangii (Kachalkin et al.) Schoutteten. Citation: Schoutteten N, Yurkov A, Spirin V, Savchenko A, Aime MC, Begerow D, Verbeken A (2024). Examination of mycoparasites reveals a new type of host-parasite interface and rearranges the taxonomy of Occultifur and Microsporomyces (Cystobasidiomycetes, Basidiomycota). Studies in Mycology 109: 451-486. doi: 10.3114/sim.2024.109.07.
Keywords: Basidiomycota; dimorphism; fungal systematics; haustorial cells; host-parasite interaction; molecular phylogeny; mycoparasitism; new taxa; taxonomy; transmission electron microscopy; yeast.
© 2024 Westerdijk Fungal Biodiversity Institute.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures











References
-
- Aime MC, Toome M, McLaughlin DJ. (2014). Pucciniomycotina. In: The Mycota VII. Systematics and evolution. Part A. (McLaughlin DJ, Spatafora JW, eds). Springer, Germany: 271–294.
-
- Akulov OY, Fomenko MI, Khudych AS. et al. (2022). The first find of Naohidea sebacea (Naohideales, Basidiomycota) in Ukraine. Ukrainian Botanical Journal 79(5): 308–313.
-
- Bai FY, Cai Y, Wang QM. et al. (2004). Rhodotorula oryzae sp. nov., a novel basidiomycetous yeast species isolated from paddy rice. Antonie van Leeuwenhoek 86: 295–299. - PubMed
-
- Bai F, Liu Y, Li N. et al. (2016). Microsporomyces hainanensis sp. nov., isolated from hybrid rice (Oryza sativa L.) seeds. Current Microbiology 73: 569–573. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
