Multidimensional insights into exosomes in hepatocellular carcinoma: from genesis to clinical application
- PMID: 40881702
- PMCID: PMC12380686
- DOI: 10.3389/fimmu.2025.1628573
Multidimensional insights into exosomes in hepatocellular carcinoma: from genesis to clinical application
Abstract
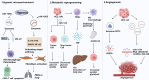
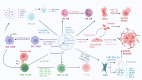
Hepatocellular carcinoma (HCC) is a highly aggressive malignancy, whose progression is intimately linked to the complex dynamics of the tumor microenvironment (TME). Exosomes, once considered mere cellular waste, have emerged as pivotal mediators of intercellular communication within the TME, actively participating in the multistep development of HCC. These nanoscale vesicles play crucial roles in the initiation of precancerous lesions and, by transporting drug resistance-related molecules such as proteins and non-coding RNAs, facilitate the acquisition of resistance to chemotherapy and targeted therapies by tumor cells. Moreover, exosomes contribute to the establishment of pre-metastatic niches by remodeling distant organ microenvironments-inducing hypoxia, metabolic reprogramming, and angiogenesis-which collectively create favorable conditions for tumor cell colonization. They also modulate immune responses by inducing T-cell exhaustion, promoting macrophage polarization, and disrupting normal stromal cell functions, thereby constructing an immunosuppressive microenvironment that enables tumor immune evasion. Given their inherent biocompatibility and targeting capabilities, engineered exosomes have shown promise in cancer therapy, serving as carriers for therapeutic molecules and enabling precise drug delivery through surface modifications. Despite significant advancements, challenges remain in elucidating the in vivo regulatory mechanisms of exosomes, standardizing their isolation and purification processes, and evaluating their clinical efficacy. This review examines the multifaceted roles of exosomes in HCC, aiming to bridge mechanistic insights with precision diagnostics and pave new avenues for liver cancer treatment.
Keywords: drug tolerance; exosomes; hepatocellular carcinoma (HCC); metastasis; targeted therapy; tumor microenvironment (TME).
Copyright © 2025 Zhang, Zhang, Zhang and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

