A phase II study of intrapatient dose escalation of biweekly trifluridine/tipiracil plus bevacizumab for colorectal cancer (E-BiTS study)
- PMID: 40882248
- PMCID: PMC12414899
- DOI: 10.1016/j.esmoop.2025.105571
A phase II study of intrapatient dose escalation of biweekly trifluridine/tipiracil plus bevacizumab for colorectal cancer (E-BiTS study)
Abstract
Background: Trifluridine/tipiracil (FTD/TPI) plus bevacizumab (BEV) remains a standard of care for refractory metastatic colorectal cancer (mCRC), though hematological toxicities remain a concern. Although a biweekly regimen has shown favorable tolerability, the optimal biweekly dosage has yet to be established. Thus, this study was conducted to evaluate the feasibility and clinical impact of intrapatient dose escalation of biweekly FTD/TPI plus BEV.
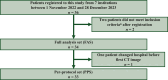
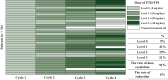
Materials and methods: This multicenter, single-arm phase Ⅱ trial included patients with refractory mCRC who had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0-1. Eligible patients received biweekly FTD/TPI (35 mg/m2 twice daily on days 1-5, every 2 weeks) and BEV (5 mg/kg on day 1). The FTD/TPI dose could be escalated up to 30 mg/day based on tolerability during cycles 2-4. The primary endpoint was disease control rate, whereas the secondary endpoints included progression-free survival (PFS), overall survival (OS), relative dose intensity, and safety.
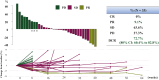
Results: Among the 36 enrolled patients, 34 were included in the full analysis set. The median age was 65 years (range 39-79 years); 76% had ECOG PS 0, 56% had RAS mutations, and 79% had two or more metastatic organs. Dose escalation was feasible in 91% of the patients. The disease control rate was 72.7% (80% confidence interval 60.4% to 82.8%), median PFS was 5.6 months, and median OS was 17.6 months, with a median follow-up duration of 18.0 months. Patients who experienced grade ≥2 neutropenia had significantly improved outcomes (PFS, 7.0 versus 2.3 months; OS, not reached versus 10.6 months). Hematologic toxicities were manageable, with no febrile neutropenia and only three treatment-related serious adverse events.
Conclusions: Intrapatient dose escalation of biweekly FTD/TPI plus BEV was feasible and well tolerated and may enhance the efficacy of refractory mCRC treatment. Nonetheless, further studies are warranted to validate this individualized dosing strategy.
Keywords: biweekly trifluridine/tipiracil plus bevacizumab; colorectal cancer; intrapatient dose escalation strategy.
Copyright © 2025 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Mayer R.J., Cutsem E.V., Falcone A., et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372(20):1909–1919. - PubMed
-
- Prager G.W., Taieb J., Fakih M., et al. Trifluridine-tipiracil and bevacizumab in refractory metastatic colorectal cancer. N Engl J Med. 2023;388(18):1657–1667. - PubMed
-
- Matsuoka H., Yamada T., Ohta R., et al. Biweekly TAS-102 and bevacizumab as third-line chemotherapy for advanced or recurrent colorectal cancer: a phase II, multicenter, clinical trial (TAS-CC4 study) Int J Clin Oncol. 2022;27(12):1859–1866. - PubMed
-
- Satake H., Yamazaki K., Suwa Y., et al. First report of the randomized phase III study of bi-weekly trifluridine/tipiracil (FTD/TPI) plus bevacizumab (BEV) vs. FTD/TPI monotherapy for chemorefractory metastatic colorectal cancer (mCRC): JCOG2014 (ROBiTS) J Clin Oncol. 2024;42(suppl 3):118.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

