Proanthocyanidins inhibit CYP1B1 through mixed-type kinetics and stable binding in molecular dynamics simulations
- PMID: 40883330
- PMCID: PMC12397248
- DOI: 10.1038/s41598-025-12781-2
Proanthocyanidins inhibit CYP1B1 through mixed-type kinetics and stable binding in molecular dynamics simulations
Abstract
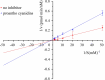
Cytochrome P450 1B1 (CYP1B1) is a heme-containing enzyme involved in procarcinogen activation and estrogen metabolism, contributing to tumor progression. This study investigates the inhibitory effects of proanthocyanidin (PA) on CYP1B1-catalyzed reactions and its underlying mechanisms. Enzyme kinetics revealed that PA exerts mixed-type inhibition with an IC₅₀ of 2.53 ± 0.01 μM. Molecular docking demonstrated that PA binds to key residues (Phe231, Gly329, Ala330, Asn228, Asn265) and the heme cofactor through hydrogen bonding and π-π stacking, interfering with substrate binding and electron transfer. Molecular dynamics simulations over 200 ns confirmed the stability of the PA-CYP1B1 complex. To validate the stability and inhibitory relevance of the simulation results, berberine, a known CYP1B1 inhibitor, was used as a positive control in parallel analyses. In silico ADMET prediction indicated high intestinal absorption and a favorable safety profile, with no significant CYP inhibition or mutagenicity. However, low membrane permeability and multiple drug-likeness violations suggest limited oral bioavailability. These findings support the potential of PA as a natural CYP1B1 inhibitor for cancer prevention and treatment. Further structural optimization or formulation strategies may enhance its pharmacokinetic properties and clinical applicability.
Keywords: ADMET; CYP1B1; Inhibition kinetics; Molecular dynamics; Proanthocyanidin.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures








Similar articles
-
Discovery and synthesis of azepinoindoles as novel hCYP1B1 inhibitors with AhR-independent anticancer activity.Bioorg Chem. 2025 Aug;163:108786. doi: 10.1016/j.bioorg.2025.108786. Epub 2025 Jul 21. Bioorg Chem. 2025. PMID: 40706542
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Discovery of Highly Selective CYP1B1 Inhibitors.J Med Chem. 2025 Jun 26;68(12):13089-13112. doi: 10.1021/acs.jmedchem.5c01181. Epub 2025 Jun 16. J Med Chem. 2025. PMID: 40522756
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

