Comparative effects of focused ultrasound and microbubbles on healthy and tumor bearing rat spinal cord
- PMID: 40883385
- PMCID: PMC12397393
- DOI: 10.1038/s41598-025-13722-9
Comparative effects of focused ultrasound and microbubbles on healthy and tumor bearing rat spinal cord
Abstract
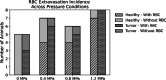
This study examined the differential sensitivity of intramedullary spinal cord tumors (IMSCTs) and healthy tissue to focused ultrasound (FUS) and microbubbles in a rat model of IMSCT. F98 glioma cells were injected into spinal cords of F344 rats. FUS (580 kHz, 10 ms bursts, 1 Hz pulse repetition frequency, 40 s) was delivered to tumor and adjacent healthy tissue at varying pressures (0-1.2 MPa) following intravenous injection of microbubbles (1.00 ± 0.85 µm; 2.4 × 107 microbubbles/100 g). Tissues were collected 24 h post-treatment for histological analysis. Healthy tissue exhibited pressure-dependent damage, including significant differences in red blood cell (RBC) extravasation between 0 and 1.2 MPa conditions (0 ± 0 vs 1.73 × 105 ± 2.15 × 105, p = 0.015), hemorrhagic pools, and tissue disintegration. Conversely, the presence of histopathological features in tumors, regardless of pressure, and no significant differences in RBC extravasation areas between exposure conditions suggests no treatment-induced damage at the tested exposures. These findings indicate F98 gliomas are less sensitive to FUS and microbubbles than healthy spinal cord, likely due to reduced vascularity (p < 0.00001 compared to grey matter, p < 0.05 compared to white matter). This finding indicates alternative strategies (e.g. nanodroplets or molecularly-targeted bubbles) must be explored for effectively treating CNS tumors with low vascularity.
Keywords: Anti-vascular therapy; Focused ultrasound; Glioma; Microbubble; Spinal cord tumor; Tumor vasculature.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: M.A.O. holds industry partnered funding (Ontario Research Fund) with FUS Instruments as an industry partner. The other authors declare no competing interests.
Figures










References
-
- Dijkmans, P. A. et al. Microbubbles and ultrasound: From diagnosis to therapy. Eur. J. Echocardiogr.5, 245–246 (2004). - PubMed
-
- Holland, C. K. & Apfel, R. E. Fundamentals of the mechanical index and caveats in its application. J. Acoust. Soc. Am.105, 1324 (1999).
-
- Chowdhury, S. M., Abou-Elkacem, L., Lee, T., Dahl, J. & Lutz, A. M. Ultrasound and microbubble mediated therapeutic delivery: Underlying mechanisms and future outlook. J. Control. Release326, 75–90 (2020). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

