Photocatalytic degradation of Rhodamine B dye over Ni-Cd doped and co-doped ZnO nanoparticles
- PMID: 40883394
- PMCID: PMC12397293
- DOI: 10.1038/s41598-025-17684-w
Photocatalytic degradation of Rhodamine B dye over Ni-Cd doped and co-doped ZnO nanoparticles
Abstract
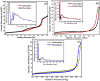
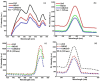
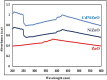
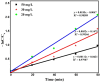
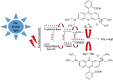
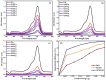
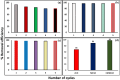
This study demonstrates the photocatalytic degradation efficiency of doped NiZnO and co-doped CdNiZnO NPs. Initially, ZnO NPs with a unique mesoporous ellipsoidal morphology were synthesized by simple precipitation and calcination. Powder X-ray diffraction revealed the formation of a hexagonal phase of the wurtzite structure. The average crystallite size of pristine ZnO NPs is 48 nm. The NPs possess higher thermal stability with the surface area, pore volume, and pore size of 9.1302 m2/g, 0.028299 cm3/g, and 12.39819 nm, respectively. Furthermore, different mesoporous doped NiZnO and co-doped CdNiZnO NPs in the range of 34 and 29 nm were synthesized by co-precipitation method and characterized by XRD, EDX, SEM, TEM, PL, Raman, BET analyses and UV-Vis spectroscopy. The calculated optical bandgaps for pure ZnO, doped NiZnO and co-doped CdNiZnO NPs were found to be 3.1, 2.62 and 2.33 eV, respectively. The photocatalytic activity of co-doped CdNiZnO was significantly higher than that of pure ZnO. After 50 min of irradiation, approximately 98% of rhodamine B was degraded by CdNiZnO, compared to 65% with pure ZnO. This enhancement is attributed to the synergistic effects of Ni and Cd, which trap electrons and holes, reducing recombination and extending charge carrier lifetimes. The photocatalytic efficiency of the synthesized materials to decompose the RhB dye (30 mgL-1) in aqueous media was tested via Langmuir-Hinshelwood model under UV-visible light. The degradation process followed pseudo-first order kinetic model. The synthesized NPs were re-used for five cycles without any significant decrease in the photodegradation ability. The mechanistic concept of generating reactive oxygen species by electron and hole charge (e‾/h+ ) carriers seems to be responsible for the photocatalytic degradation of the dye by CdNiZnO NPs. Zeta potential analysis revealed positive surface charges for all catalysts, with co-doping significantly increasing charge and colloidal stability, thereby supporting the pH-dependent photocatalytic performance.
Keywords: CdNiZnO; Co-precipitation; Photocatalysts; Pseudo first order kinetics; Rhodamine B.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures











Similar articles
-
Photocatalytic Degradation of Synozol Navy Blue Dye by Ni-Cu-Ag Dopped ZnO: An Insight Into Photoluminescence, Scavenging, Mechanism and Kinetic Aspects.Microsc Res Tech. 2025 Sep;88(9):2392-2407. doi: 10.1002/jemt.24867. Epub 2025 Mar 31. Microsc Res Tech. 2025. PMID: 40165504
-
One-step co-precipitation synthesis, characterization, and enhanced photocatalytic performance of CaO/TiO2-supported γ-Al2O3 nanocomposites (NCs) in wastewater treatment.RSC Adv. 2025 Jul 15;15(30):24851-24861. doi: 10.1039/d5ra03493k. eCollection 2025 Jul 10. RSC Adv. 2025. PMID: 40666418 Free PMC article.
-
Simulation, synthesis, and characterization of Ni-Co and its co-doping in ZnO for energy applications.RSC Adv. 2025 Jul 3;15(28):22730-22744. doi: 10.1039/d5ra02746b. eCollection 2025 Jun 30. RSC Adv. 2025. PMID: 40612632 Free PMC article.
-
Ultrasonically enhanced photocatalytic degradation of methylene blue by Nano-CoFe2O4-immobilized Saccharomyces cerevisiae yeast composite as a photo-Fenton catalyst: A central composite design study.Int J Biol Macromol. 2025 Sep;322(Pt 4):147068. doi: 10.1016/j.ijbiomac.2025.147068. Epub 2025 Aug 22. Int J Biol Macromol. 2025. PMID: 40850420 Review.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
References
-
- Mohanty, L., Pattanayak, D. S., Singhal, R., Pradhan, D. & Dash, S. K. Enhanced photocatalytic degradation of rhodamine B and malachite green employing BiFeO3/g-C3N4 nanocomposites: An efficient visible-light photocatalyst. Inorg. Chem. Commun.138, 109286 (2022).
-
- Byrappa, K. et al. Photocatalytic degradation of rhodamine B dye using hydrothermally synthesized ZnO. Bull. Mater. Sci.29, 433–438 (2006).
-
- Liu, S. et al. The influence of metal organic frameworks(MOFs) after tannic acid etching on the performance and structure of loose nanofiltration membranes for enhanced dyes/salts selective separation. J. Water Process Eng.70, 107009 (2025).
Grants and funding
- Grant No. KFU252985/Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University Saudi Arabia
- Grant No. KFU252985/Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University Saudi Arabia
LinkOut - more resources
Full Text Sources

