DNMT1 recruits RUNX1 and represses FOXO1 transcription to inhibit anti-inflammatory activity of regulatory T cells and augments sepsis-induced lung injury
- PMID: 40883460
- PMCID: PMC12397193
- DOI: 10.1007/s10565-025-10069-9
DNMT1 recruits RUNX1 and represses FOXO1 transcription to inhibit anti-inflammatory activity of regulatory T cells and augments sepsis-induced lung injury
Abstract
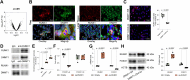
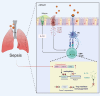
Sepsis-induced lung injury (ALI) is a critical condition characterized by excessive immune responses and tissue damage. Previous evidence has underscored an upregulation pattern of DNA methyltransferase 1 (DNMT1) in sepsis. This study reveals the key role of DNMT1 in modulating regulatory T cell (Treg) activity in septic ALI. A septic mouse model was generated through cecal ligation and puncture. Treatment with either DNMT1 antagonist Thioguanine (ThG) or AAV-sh-DNMT1 significantly reduced immune cell infiltration, reduced production of pro-inflammatory cytokines, and increasing production of anti-inflammatory cytokines in the bronchoalveolar lavage fluid (BALF) of mice, alongside improved lung pathology and integrity. Furthermore, the DNMT1 inhibition or silencing significantly enhanced population of FOXP3+ Tregs in the BALF and lung tissue. Similar trends were observed in mice with specific DNMT1 deletion in CD4+ T cells (DNMT1-CD4-ko). Regarding the mechanism, we observed that DNMT1 represses transcription of forkhead box O1 (FOXO1) by recruiting RUNX family transcription factor 1 (RUNX1) to the FOXO1 promoter. FOXO1-specific knockout in CD4+ T cells reduced anti-inflammatory activity of Tregs. Additionally, administration of the CD25 antibody exacerbated sepsis-induced ALI in DNMT1-CD4-ko mice. Collectively, these findings illustrate that targeting DNMT1 interacts with RUNX1 to repress transcription of FOXO1, which reduces immunomodulatory activity of Tregs and augments inflammatory cascades in septic lung injury.
Keywords: DNMT1; FOXO1; Lung injury; RUNX1; Sepsis; Tregs.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Shanghai Pulmonary Hospital Ethics Committee. All animal experiments complied with the guidelines of the Shanghai Pulmonary Hospital Animal Care and Use Committee (Approval number: K21-354). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures






Similar articles
-
orexin B alleviates sepsis-associated lung injury through the attenuation of pulmonary endothelial barrier dysfunction by regulating the rho-associated coiled-coil containing protein kinase 2/zonula occludens-1 (ROCK2/ZO-1) axis.Biotechnol Appl Biochem. 2025 Aug;72(4):883-896. doi: 10.1002/bab.2703. Epub 2024 Dec 2. Biotechnol Appl Biochem. 2025. PMID: 39623599
-
Diosgenin Improves Lipid Metabolism in Diabetic Nephropathy via Regulation of miR-148b-3p/DNMT1/FOXO1 Axis.Nephron. 2025;149(4):226-239. doi: 10.1159/000541690. Epub 2024 Nov 27. Nephron. 2025. PMID: 39602888
-
Macrophage TRIM21 knockout inhibits septic acute lung injury by downregulating autophagy regulator protein ubiquitination.Autophagy. 2025 Jun 24:1-20. doi: 10.1080/15548627.2025.2519063. Online ahead of print. Autophagy. 2025. PMID: 40509575
-
Therapeutic impact of human trophoblast stem cells in peritoneal and pneumonia-induced sepsis in mice.Stem Cell Res Ther. 2025 Jul 21;16(1):394. doi: 10.1186/s13287-025-04479-z. Stem Cell Res Ther. 2025. PMID: 40691621 Free PMC article.
-
The Multifaceted Role of Regulatory T Cells in Sepsis: Mechanisms, Heterogeneity, and Pathogen-Tailored Therapies.Int J Mol Sci. 2025 Aug 1;26(15):7436. doi: 10.3390/ijms26157436. Int J Mol Sci. 2025. PMID: 40806563 Free PMC article. Review.
References
-
- Carrette F, Fabre S, Bismuth G. FOXO1, T-cell trafficking and immune responses. Adv Exp Med Biol. 2009;665:3–16. 10.1007/978-1-4419-1599-3_1. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

