Nanofat promotes wound healing in skin following exposure to ionizing radiation
- PMID: 40883558
- PMCID: PMC12397309
- DOI: 10.1038/s41598-025-17961-8
Nanofat promotes wound healing in skin following exposure to ionizing radiation
Abstract
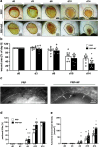
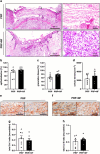
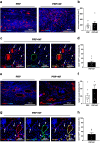
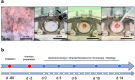
Radiotherapy, while effective in cancer treatment, can lead to side effects, such as radiodermatitis with potential long-term consequences including telangiectasias, ulceration and fibrosis of the skin, eventually resulting in impaired wound healing. In this study, we analyzed whether the healing of such challenging wounds can be improved by nanofat (NF). NF is generated by mechanical emulsification and filtration of fat samples and, thus, is a random mixture of adipose-derived stem cells, microvascular fragments, extracellular matrix components and growth factors. Two months after localized ionizing radiation of the skin with a total dose of 20 Gy, full-thickness wounds were created in dorsal skinfold chambers of mice, which were filled with platelet-rich plasma (PRP; control, n = 8) or NF fixed in PRP (PRP + NF, n = 8). The healing process was assessed by means of stereomicroscopy, intravital fluorescence microscopy, histology and immunohistochemistry over 14 days. The closure of PRP + NF-treated wounds was accelerated, as indicated by significantly smaller wound areas on day 14 when compared to controls. This was associated with a higher density of blood-perfused microvessels inside the wounds. Moreover, PRP + NF-treated wounds showed a tendency towards an improved granulation tissue formation, lymphatic drainage and M2/M1 macrophage ratio. Taken together, these findings suggest that the application of NF represents a promising therapeutic strategy for the management of complex wounds in irradiated skin.
Keywords: Dorsal skinfold chamber; Nanofat; Platelet-rich plasma; Radiotherapy; Vascularization; Wound healing.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


References
-
- Jensen, J. M. & Proksch, E. The skin’s barrier. G Ital. Dermatol. Venereol.144, 689–700 (2009). - PubMed
-
- Wang, P. H., Huang, B. S., Horng, H. C., Yeh, C. C. & Chen, Y. J. Wound healing. J. Chin. Med. Assoc.81, 94–101 (2018). - PubMed
-
- Russi, E. G. et al. Acute skin toxicity management in head and neck cancer patients treated with radiotherapy and chemotherapy or EGFR inhibitors: literature review and consensus. Crit. Rev. Oncol. Hematol.96, 167–182 (2015). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

