Pyrin inflammasome-driven erosive arthritis caused by unprenylated RHO GTPase signaling
- PMID: 40883609
- PMCID: PMC12514176
- DOI: 10.1038/s44321-025-00298-0
Pyrin inflammasome-driven erosive arthritis caused by unprenylated RHO GTPase signaling
Abstract
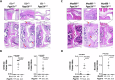
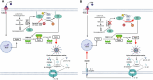
Geranylgeranyl pyrophosphate, a non-sterol intermediate of the mevalonate pathway, serves as the substrate for protein geranylgeranylation, a process catalyzed by geranylgeranyl transferase I (GGTase-I). Myeloid-specific deletion of Pggt1b, the gene coding for GGTase-I, leads to spontaneous and severe erosive arthritis in mice; however, the underlying mechanisms remained unclear. In this study, we demonstrate that arthritis in mice with myeloid-specific Pggt1b deficiency is driven by unprenylated GTP-bound small RHO family GTPases, which in turn trigger Pyrin (Mefv) inflammasome activation, GSDMD-dependent macrophage pyroptosis, and IL-1β secretion. We show that although Pggt1b deficiency leads to hyperactivation of RAC1, impaired prenylation alters its proper membrane localization and interaction with effectors, rendering it effectively inactive in vivo. Consequently, unprenylated RHO family signaling promotes Pyrin inflammasome assembly through recruitment to the RAC1 effector IQGAP1. Together, these findings identify a novel inflammatory axis in which non-prenylated RHO GTPase activity promotes spontaneous Pyrin inflammasome activation, pyroptosis, and IL-1β release in macrophages, contributing to inflammatory arthritis in mice.
Keywords: Arthritis; Prenylation; Pyrin Inflammasome; Pyroptosis; RHO Family GTPases.
© 2025. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. The authors declare no competing interests.
Figures












References
-
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S (1998) Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143–150 - PubMed
-
- An C, Sun F, Liu C, Huang SJ, Xu T, Zhang CX, Ge SL (2023) IQGAP1 promotes mitochondrial damage and activation of the mtDNA sensor cGAS-STING pathway to induce endothelial cell pyroptosis leading to atherosclerosis. Int Immunopharmacol 123:110795 - PubMed
MeSH terms
Substances
Grants and funding
- 2020-06494/Vetenskapradet
- WGF2021-0085/Wenner-Gren Stiftelserna (Wenner-Gren Foundations)
- BOF/24J/2021/052/UGent | Bijzonder Onderzoeksfonds UGent (BOF)
- 01G00123/UGent | Bijzonder Onderzoeksfonds UGent (BOF)
- BOF.24Y.2019.0032.01/UGent | Bijzonder Onderzoeksfonds UGent (BOF)
- 3G090322/Fonds Wetenschappelijk Onderzoek (FWO)
- 3G0H2522/Fonds Wetenschappelijk Onderzoek (FWO)
- GOI5722N/Fonds Wetenschappelijk Onderzoek (FWO)
- G017121N/Fonds Wetenschappelijk Onderzoek (FWO)
- G014221N/Fonds Wetenschappelijk Onderzoek (FWO)
- 3G044718/Fonds Wetenschappelijk Onderzoek (FWO)
- 3G044818/Fonds Wetenschappelijk Onderzoek (FWO)
- G0A3422N/Fonds Wetenschappelijk Onderzoek (FWO)
- G0A7O24N/Fonds Wetenschappelijk Onderzoek (FWO)
- 101101075/EC | European Research Council (ERC)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

