Impact of artificial liver plasma perfusion combined with plasma exchange therapy on clinical efficacy and short-term prognosis of hepatitis B virus-related acute-on-chronic liver failure with or without HIV infection
- PMID: 40883671
- PMCID: PMC12398131
- DOI: 10.1186/s12879-025-11496-z
Impact of artificial liver plasma perfusion combined with plasma exchange therapy on clinical efficacy and short-term prognosis of hepatitis B virus-related acute-on-chronic liver failure with or without HIV infection
Abstract
Objective: To compare the effectiveness of plasma perfusion combined with plasma exchange (PP + PE) artificial liver support system in patients with hepatitis B virus-associated acute-on-chronic liver failure with and without HIV infection (HBV-ACLF/HIV(+) and HBV-ACLF/HIV(-), respectively) and to assess the clinical value and safety of the artificial liver support system.
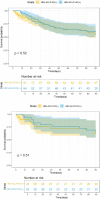
Methods: This study involved 162 patients diagnosed with HBV-ACLF and hospitalized at Chengdu Public Health Clinical Medical Center from January 2020 to January 2025, in accordance with the diagnostic criteria of the Chinese Guidelines for the Diagnosis and Treatment of Acute-on-Chronic Liver Failure (2025 edition). Seventeen patients with tumors, severe underlying diseases, or other hepatitis virus infections were excluded. Patients were divided into the HBV-ACLF/HIV(+) and HBV-ACLF/HIV(-) groups. Propensity score matching (PSM) was used to correct baseline bias. Laboratory indices, Model for End-stage Liver Disease score (MELDs), and 28- and 90-day cumulative survival or mortality rates before and after artificial liver therapy were used as effectiveness indicators. Kaplan-Meier analysis was used to plot survival curves, and the log rank test was used to assess survival differences. Cox proportional hazards regression modeling was used for multifactorial analysis to further evaluate the effectiveness of PP + PE therapy and prognostic factors.
Results: In total, 145 patients with HBV-ACLF were included, with 64 in the HBV-ACLF/HIV(+) group and 81 in the HBV-ACLF/HIV(-) group. After 1:1 PSM, 29 patients remained in each group. Alanine aminotransferase, glutamate transferase, total bilirubin, indirect bilirubin, international normalized ratio, prothrombin time activity, and MELDs significantly improved in both groups after PP + PE treatment (P < 0.05). Before and after PSM, there was no statistically significant difference in 28- or 90-day cumulative survival between the HBV-ACLF/HIV(+) and HBV-ACLF/HIV(-) groups (after PSM: log rank, P = 0.572). Multifactorial Cox regression analysis showed that improvements in total bilirubin, creatinine, leukocytes, blood ammonia, MELDs, international normalized ratio, and prothrombin time activity were associated with better short-term prognosis in patients with HBV-ACLF/HIV(+) (P < 0.005).
Conclusion: The PP + PE artificial liver treatment model effectively promotes liver function recovery and improves clinical status in HBV-ACLF patients, including those with HIV co-infection. This provides a valuable basis for clinical management of co-infected cases. Multicenter prospective studies are needed to confirm its long-term efficacy.
Keywords: Acute-on-chronic liver failure; Artificial liver support system; Clinical efficacy; Hepatitis b virus; Human immunodeficiency virus; Plasma exchange; Plasma perfusion; Short-term prognosis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study is a retrospective experiment. Trial registration number: MR-51-25-020710.Data of registration 2025.3.11. Ethical approval for this study was obtained from the Public Health Clinical Center of Chengdu. The study was a retrospective analysis, and the ethical approval was granted under the approval number [YJ-K2024-72-01]. Given the observational nature of this study, where no interventions were made and data were collected from existing records, informed consent was not required. All data were anonymized to ensure patient confidentiality, and the study was conducted in accordance with ethical guidelines and regulatory standards to protect participants’ rights. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Wang ZQ, Yang M, Chen ZJ. Detection and analysis of co-infection with HBV, HCVor Treponema Pallidum in HIV/AIDS patients. Pract Prev Med 2012. 2012;19(10):1560–2. 10.3969/j.issn.1006-3110.2012.10.046. - DOI
-
- Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Expert consensus on clinical application of artificial liver and blood purification (2022 edition). J Clin Hepatol. 2022;38(4):767–75. 10.3969/j.issn.1001-5256.2022.04.007. - DOI
-
- Chen T, Yang Z, Choudhury AK, et al. Complications constitute a major risk factor for mortality in hepatitis B virus-related acute-on-chronic liver failure patients: a multi-national study from the Asia-Pacific region. Hepatol Int. 2019;13:695–705. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous