Multi-ancestry meta-analysis identifies genetic modifiers of age-at-onset of Alzheimer's disease at known and novel loci
- PMID: 40883957
- PMCID: PMC12397202
- DOI: 10.1002/alz.70489
Multi-ancestry meta-analysis identifies genetic modifiers of age-at-onset of Alzheimer's disease at known and novel loci
Abstract
Introduction: Much of Alzheimer's disease (AD) risk is explained by age, apolipoprotein E (APOE) genotype, and sex. We sought to identify genetic modifiers of age at onset (AAO) of AD while probing the influence of sex and APOE among those with diverse ancestry.
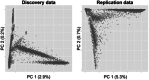
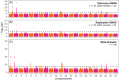
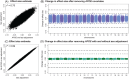
Methods: We performed genome-wide association studies (GWASs) of AAO in two diverse samples followed by meta-analysis, contrasting results with and without adjustment for sex and APOE. Genome-wide significance was set to p < 5×10-8.
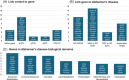
Results: GWASs adjusting for sex, APOE, population structure, and relatedness revealed 17 significant loci including independent associations at AD risk loci and four novel signals. APOE adjustment influenced GWAS effect sizes across the genome while sex adjustment had minimal effect.
Discussion: We identified association signals within a diverse but relatively small sample, replicating loci recently discovered in large European ancestry-only GWASs, and illustrated the power of using a quantitative trait like AAO over a binary diagnosis trait.
Highlights: Survival analysis approach identified known and novel genetic modifiers of Alzheimer's disease (AD). Multi-ancestry analyses revealed independent signals at known AD loci. Apolipoprotein E adjustment influenced variant effects across the genome.
Keywords: age at onset; apolipoprotein E; diversity; multi‐ancestry; sex differences.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors report no disclosures relevant to the manuscript. Author disclosures are available in the supporting information.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- P30 AG066506/AG/NIA NIH HHS/United States
- R01AG032990/National Institutes of Health (NIH) National Institute on Aging (NIA)
- P01 AG026276/AG/NIA NIH HHS/United States
- Neurotrack Technologies
- Life Science Database Integration Project of Japan Science and Technology Agency
- P30 AG066444/AG/NIA NIH HHS/United States
- Johnson & Johnson Pharmaceutical Research &Development LLC
- BioClinica
- P30 AG066518/AG/NIA NIH HHS/United States
- P30AG008017/National Institutes of Health (NIH) National Institute on Aging (NIA)
- EuroImmun
- P30 AG072958/AG/NIA NIH HHS/United States
- P30 AG066530/AG/NIA NIH HHS/United States
- P30 AG072977/AG/NIA NIH HHS/United States
- P30 AG072947/AG/NIA NIH HHS/United States
- R01NS080820/NIH/National Institute for Neurological Disorders and Stroke (NINDS)
- P50AG016574/National Institutes of Health (NIH) National Institute on Aging (NIA)
- U01AG052410/National Institutes of Health (NIH) National Institute on Aging (NIA)
- Translational Research Promotion Project from the New Energy and Industrial Technology Development Organization of Japan
- RF1AG051504/National Institutes of Health (NIH) National Institute on Aging (NIA)
- CAPMC/ CIHR/Canada
- P30 AG066514/AG/NIA NIH HHS/United States
- U24 AG021886/AG/NIA NIH HHS/United States
- U01 AG049507/AG/NIA NIH HHS/United States
- U24 AG21886/National Institutes of Health (NIH) National Institute on Aging (NIA)
- P30 AG079280/National Institutes of Health (NIH) National Institute on Aging (NIA)
- Alzheimer's Drug Discovery Foundation
- Meso Scale Diagnostics, LLC
- R01 AG064877/AG/NIA NIH HHS/United States
- Lumosity
- P30 AG062421/AG/NIA NIH HHS/United States
- Bristol-Myers Squibb Company
- Piramal Imaging
- Takeda Pharmaceutical Company
- P30 AG072931/AG/NIA NIH HHS/United States
- P30 AG072979/AG/NIA NIH HHS/United States
- ALZ/Alzheimer's Association/United States
- U01 AG082655/National Institutes of Health (NIH) National Institute on Aging (NIA)
- P30 AG062715/AG/NIA NIH HHS/United States
- P30 AG062677/AG/NIA NIH HHS/United States
- U19 AG024904/AG/NIA NIH HHS/United States
- P30 AG072946/AG/NIA NIH HHS/United States
- U01AG046139/National Institutes of Health (NIH) National Institute on Aging (NIA)
- Araclon Biotech
- R01AG048927/National Institutes of Health (NIH) National Institute on Aging (NIA)
- R01 AG054060/AG/NIA NIH HHS/United States
- Banner Alzheimer's Foundation
- P30 AG066512/AG/NIA NIH HHS/United States
- U19AG033655/National Institutes of Health (NIH) National Institute on Aging (NIA)
- P01AG03949/National Institutes of Health (NIH) National Institute on Aging (NIA)
- Novartis Pharmaceuticals Corporation
- P30 AG062429/AG/NIA NIH HHS/United States
- CereSpir, Inc.
- R01 AG030653/AG/NIA NIH HHS/United States
- RF1AG015473/National Institutes of Health (NIH) National Institute on Aging (NIA)
- GE Healthcare
- P01 AG03991/National Institutes of Health (NIH) National Institute on Aging (NIA)
- R01AG009956/National Institutes of Health (NIH) National Institute on Aging (NIA)
- P30 AG072978/AG/NIA NIH HHS/United States
- P30 AG066468/AG/NIA NIH HHS/United States
- R01 AG028786/AG/NIA NIH HHS/United States
- P30 AG086404/AG/NIA NIH HHS/United States
- P30 AG062422/AG/NIA NIH HHS/United States
- R21 AG089267/AG/NIA NIH HHS/United States
- AbbVie
- R01AG026916/National Institutes of Health (NIH) National Institute on Aging (NIA)
- Transition Therapeutics
- U01 AG032984/AG/NIA NIH HHS/United States
- P30 AG066508/AG/NIA NIH HHS/United States
- R01 AG059737/AG/NIA NIH HHS/United States
- U01 AG058589/AG/NIA NIH HHS/United States
- P30 AG066519/AG/NIA NIH HHS/United States
- U19AG06656/National Institutes of Health (NIH) National Institute on Aging (NIA)
- Cogstate
- P50 AG005136/AG/NIA NIH HHS/United States
- P30 AG072959/AG/NIA NIH HHS/United States
- P30 AG072975/AG/NIA NIH HHS/United States
- P30 AG086401/AG/NIA NIH HHS/United States
- EB/NIBIB NIH HHS/United States
- P30 AG066546/AG/NIA NIH HHS/United States
- U24AG041689/National Institutes of Health (NIH) National Institute on Aging (NIA)
- P30 AG066509/AG/NIA NIH HHS/United States
- Pfizer Inc.
- R01 AG019085/AG/NIA NIH HHS/United States
- Elan Pharmaceuticals, Inc.
- F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.
- GlaxoSmithKline
- P30 AG066511/AG/NIA NIH HHS/United States
- Eli Lilly and Company
- R01 AG027944/AG/NIA NIH HHS/United States
- P30 AG072973/AG/NIA NIH HHS/United States
- Texas Council on Alzheimer's Disease and Related Disorders
- RF1 AG061351/AG/NIA NIH HHS/United States
- R01 AG041232/AG/NIA NIH HHS/United States
- P30 AG072976/AG/NIA NIH HHS/United States
- IXICO Ltd.
- NeuroRx Research
- P30 AG066462/AG/NIA NIH HHS/United States
- Merck & Co., Inc.
- P30 AG066507/AG/NIA NIH HHS/United States
- Janssen Alzheimer Immunotherapy Research & Development, LLC
- P30 AG066515/AG/NIA NIH HHS/United States
- U19 AG066567/AG/NIA NIH HHS/United States
- U01 AG058654/AG/NIA NIH HHS/United States
- U24 AG072122/AG/NIA NIH HHS/United States
- Research on Dementia, Health Labor Sciences Research Grant
- R01AG020688/National Institutes of Health (NIH) National Institute on Aging (NIA)
- P30 AG072972/AG/NIA NIH HHS/United States
- P20 AG068082/AG/NIA NIH HHS/United States
- Eisai Inc.
- P30AG10161/National Institutes of Health (NIH) National Institute on Aging (NIA)
- U54AG052427/National Institutes of Health (NIH) National Institute on Aging (NIA)
- P50 AG05681/National Institutes of Health (NIH) National Institute on Aging (NIA)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

