Diagnosing abdominal neoplasms using a T2 mapping radial turbo spin-echo technique with partial volume correction
- PMID: 40884612
- PMCID: PMC12414507
- DOI: 10.1007/s00330-025-11931-4
Diagnosing abdominal neoplasms using a T2 mapping radial turbo spin-echo technique with partial volume correction
Abstract
Objective: T2 mapping allows for the classification of focal liver lesions, differentiating malignancies from the most common benign liver lesions, hemangiomas, and bile duct hamartomas (BDH). Partial volume (PV) due to the presence of liver and lesion within the same voxel confounds the classification of small lesions. Our objective is to develop a robust two-component T2 estimation technique (SEPG2-SP) to enable accurate T2 estimation in the presence of PV.
Materials and methods: T2 estimation accuracy was evaluated using computer simulations, physical phantom data, and in vivo in 27 subjects with focal liver lesions (16 males, 62.4 ± 14.3 years old; 11 females, 66.8 ± 5.8 years old) imaged at 1.5 T with a radial turbo spin-echo (RADTSE) technique. The SEPG2-SP model was compared to a single-component model, which does not account for PV. The area under the receiver operator characteristic curve (AUROC) was used to analyze lesion classification.
Results: Phantom data showed that the SEPG2-SP model had a T2 estimation error of 2-9% while the single component model had a larger error of 9-23%. Analysis of in vivo data from 68 focal liver lesions (33 malignancies, 7 hemangiomas, and 28 BDH) showed that the SEPG2-SP model classified all lesions correctly (AUROC = 1), regardless of their size. On the other hand, with the single-component model, there was overlap between malignancies and benign lesions driven by misclassification of hemangiomas as malignancies (AUROC = 0.84).
Conclusions: The two-component T2 model improved the characterization of focal liver lesions affected by PV, yielding complete separation of malignancies from the most common benign liver lesions.
Key points: Question Partial volume effects result in T2 estimation errors that confound the classification of small focal liver lesions. Findings The proposed two-component T2 estimation technique improves T2 estimation accuracy and allows accurate characterization of focal liver lesions in the presence of partial volume. Clinical relevance The T2 mapping technique described here offers a practical and reliable approach for quantitatively classifying focal liver lesions. It enables differentiation between the most common benign liver lesions and malignancies, even in small tumors impacted by partial volume effects.
Keywords: Abdominal imaging; Partial volume effects; Radial turbo spin echo; T2 mapping.
© 2025. The Author(s).
Conflict of interest statement
Compliance with ethical standards. Guarantor: The scientific guarantor of this publication is Dr. Maria Altbach. Conflict of interest: The authors of this manuscript declare relationships with the following companies: One of the authors, Mahesh B. Keerthivasan, is an employee of Siemens Medical Solutions USA Inc. The remaining authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. Statistics and biometry: No complex statistical methods were necessary for this paper. Informed consent: Written informed consent was obtained from all subjects (patients) in this study. Ethical approval: Institutional Review Board approval was obtained. Study subjects or cohorts overlap: None. Methodology: Prospective Experimental Performed at one institution
Figures
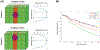



Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Does Augmenting Irradiated Autografts With Free Vascularized Fibula Graft in Patients With Bone Loss From a Malignant Tumor Achieve Union, Function, and Complication Rate Comparably to Patients Without Bone Loss and Augmentation When Reconstructing Intercalary Resections in the Lower Extremity?Clin Orthop Relat Res. 2025 Jun 26;483(9):1680-1695. doi: 10.1097/CORR.0000000000003599. Clin Orthop Relat Res. 2025. PMID: 40569278
-
[Volume and health outcomes: evidence from systematic reviews and from evaluation of Italian hospital data].Epidemiol Prev. 2013 Mar-Jun;37(2-3 Suppl 2):1-100. Epidemiol Prev. 2013. PMID: 23851286 Italian.
-
The effect of sample site and collection procedure on identification of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Dec 16;12(12):CD014780. doi: 10.1002/14651858.CD014780. Cochrane Database Syst Rev. 2024. PMID: 39679851 Free PMC article.
References
-
- Siegelman ES, Chauhan A (2014) MR characterization of focal liver lesions: pearls and pitfalls. Magn Reson Imaging Clin N Am 22:295–313 - PubMed
-
- Mulé S, Kharrat R, Zerbib P et al. (2022) Fast T2-weighted liver MRI: image quality and solid focal lesions conspicuity using a deep learning accelerated single breath-hold HASTE fat-suppressed sequence. Diagn Interv Imaging 103:479–485 - PubMed
-
- Szklaruk J, Son JB, Starr BF et al. (2020) Evaluation of feasibility and image quality of a new radial quantitative T2 weighted imaging sequence for liver MRI. Clin Imaging 66:77–81 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

