Biomarker-Based ABC-AF Risk Scores for Personalized Treatment to Reduce Stroke or Death in Atrial Fibrillation: A Registry-Based, Multicenter, Randomized, Controlled Study
- PMID: 40884774
- PMCID: PMC12643572
- DOI: 10.1161/CIRCULATIONAHA.125.076725
Biomarker-Based ABC-AF Risk Scores for Personalized Treatment to Reduce Stroke or Death in Atrial Fibrillation: A Registry-Based, Multicenter, Randomized, Controlled Study
Abstract
Background: The clinical use of risk scores to guide treatment decisions and improve clinical outcomes has rarely been prospectively evaluated. This study aimed to evaluate whether a biomarker-based ABC-AF risk score-guided multidimensional treatment strategy improves long-term outcomes in patients with AF.
Methods: The multicenter, registry-based, randomized, controlled, open-label study enrolled adults with AF. In the ABC-AF strategy arm, the investigator was informed of each individual's ABC-AF score risks for stroke and bleeding, which were used as decision support to tailor treatment recommendations, including preference for type of direct oral anticoagulant treatment. In the standard of care arm, patient management was at the discretion of the investigator. Primary outcome was a composite of stroke or death. Secondary outcomes included stroke, death, major bleeding events, and their composite outcome.
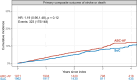
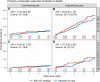
Results: The intention-to-treat population comprised 3933 patients with a median age of 73.7 years; 33.6% were women, 51.3% had paroxysmal AF, 11.2% had a previous stroke or transient ischemic attack, and 85.7% had oral anticoagulant treatment. After randomization, 97.8% in the ABC-AF strategy arm and 92.6% in the standard of care arm received OACs (P<0.0001). Enrollment was prematurely terminated owing to safety concerns with a trend toward higher mortality in patients with CHA2DS2-VASc scores of ≥3, and the study was therefore underpowered for its primary objective. Over a median follow-up of 2.6 years, 175 primary events (3.18/100 patient-years [100PY]) occurred in the ABC-AF strategy and 148 (2.67/100PY) in the standard of care arm (hazard ratio [HR], 1.19 [95% CI, 0.96-1.48]; P=0.12). Major bleeding events were 152 (2.82/100PY) versus 141 (2.61/100PY; HR, 1.08 [95% CI, 0.86-1.36]; P=0.50), stroke 48 (0.87/100PY) versus 41 (0.74/100PY; HR, 1.18 [95% CI, 0.78-1.79]; P=0.44), death 136 (2.44/100PY) versus 113 (2.02/100PY; HR, 1.21 [95% CI, 0.94-1.55]; P=0.13), and rates of composite stroke, death, or major bleeding 277 (5.21/100PY) versus 244 (4.55/100PY; HR, 1.14 [95% CI, 0.96-1.36]; P=0.13). Primary outcome results were similar across ABC-AF score subgroups (interaction P=0.98).
Conclusions: The individually tailored multidimensional treatment strategy, based on ABC-AF risk scores, did not improve clinical outcomes compared with usual guideline-based care in patients with AF. The results emphasize the need for prospective testing of the use of risk stratification and precision medicine tools in different clinical settings before implementation in routine care.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT03753490.
Keywords: atrial fibrillation; biomarkers; intracranial hemorrhages; risk factors; stroke.
Conflict of interest statement
Dr Oldgren reports institutional research grants from Amgen, AstraZeneca, Bayer, Novo Nordisk, and Roche Diagnostics; advisory board fees from Regeneron; and lecture fees from Pfizer, paid to his institution. Dr Hijazi is employed by Thermo Fisher Scientific. During the ABC-AF study, he reports research support from The Swedish Society for Medical Research (S17-0133), The Swedish Heart-Lung Foundation (20200722), and Uppsala University Hospital, Sweden; lecture/consulting fees from Bayer, Bristol-Myers Squibb, Pfizer, and Roche Diagnostics; and participation on data safety monitoring boards for Bristol-Myers Squibb and Pfizer. Dr Björkenheim reports speaker honoraria from Abbott, Bayer, and Medtronic. Dr Ravn-Fischer reports lecture fees from Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Johnson and Johnson, Lilly, Novartis, Novo Nordisk, Orion Pharma, Organon, Sanofi, and Pfizer; she is an advisory board member for Amarin, Amgen, AstraZeneca, Novartis, and Sanofi and a board member of RIKS-HIA (unpaid). Dr Själander reports lecture fees from Bayer, Bristol-Myers Squibb, and Pfizer and is chair of the Swedish Society on Thrombosis and Haemostasis. Dr Wallentin reports receiving grants and equipment and assays from Roche Diagnostics and holds 2 patents on GDF-15 (Nos. EP2047275 B1 and US8951742 B2).
Figures



Comment in
-
Time for Precision Medicine in Atrial Fibrillation Management? Not So Fast.Circulation. 2025 Nov 25;152(21):1470-1472. doi: 10.1161/CIRCULATIONAHA.125.077440. Epub 2025 Nov 24. Circulation. 2025. PMID: 41284772 No abstract available.
References
-
- Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, et al. ; ESC Scientific Document Group. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the Diagnosis and Management of Atrial Fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612 - PubMed
-
- January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. doi: 10.1161/CIR.0000000000000665 - PubMed
-
- Joglar JA, Chung MK, Armbruster AL, Benjamin EJ, Chyou JY, Cronin EM, Deswal A, Eckhardt LL, Goldberger ZD, Gopinathannair R, et al. ; Peer Review Committee Members. 2023 ACC/AHA/ACCP/HRS Guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1–e156. doi: 10.1161/CIR.0000000000001193 - PMC - PubMed
-
- Van Gelder IC, Rienstra M, Bunting KV, Casado-Arroyo R, Caso V, Crijns HJGM, De Potter TJR, Dwight J, Guasti L, Hanke T, et al. ; ESC Scientific Document Group. 2024 ESC guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2024;45:3314–3414. doi: 10.1093/eurheartj/ehae176 - PubMed
-
- Carnicelli AP, Hong H, Connolly SJ, Eikelboom J, Giugliano RP, Morrow DA, Patel MR, Wallentin L, Alexander JH, Cecilia Bahit M, et al. ; COMBINE AF (A Collaboration Between Multiple Institutions to Better Investigate Non-Vitamin K Antagonist Oral Anticoagulant Use in Atrial Fibrillation) Investigators. Direct oral anticoagulants versus warfarin in patients with atrial fibrillation: patient-level network meta-analyses of randomized clinical trials with interaction testing by age and sex. Circulation. 2022;145:242–255. doi: 10.1161/CIRCULATIONAHA.121.056355 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

