Nucleotide-dependent conformational changes direct peptide export by the transporter associated with antigen processing
- PMID: 40885191
- PMCID: PMC12422388
- DOI: 10.1016/j.immuni.2025.08.003
Nucleotide-dependent conformational changes direct peptide export by the transporter associated with antigen processing
Abstract
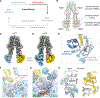
The transporter associated with antigen processing (TAP) delivers peptide antigens from the cytoplasm into the endoplasmic reticulum (ER) for loading onto major histocompatibility complex class I (MHC-I) molecules. To examine the mechanisms of peptide transport and release into the ER, we determined cryo-electron microscopy structures of the human TAP heterodimer in multiple functional states along the transport cycle. In the inward-facing conformation, when the peptide translocation cavity within the TAP heterodimer is exposed to the cytosol, ATP binding strengthened intradomain assembly. Transition to the outward-facing conformation, when the transporter opens to the ER lumen, led to a complete reconfiguration of the peptide-binding site, facilitating peptide release. ATP hydrolysis opened the catalytically active nucleotide-binding consensus site, and the subsequent separation of the nucleotide-binding domains reset the transport cycle. These findings establish a comprehensive structural framework for understanding unilateral peptide transport, vanadate trapping, and trans-inhibition-an internal feedback mechanism that prevents excessive peptide accumulation and activation of the ER stress response.
Keywords: ABC transporter; MHC-I; adaptive immunity; antigen presentation; nucleotide-binding domain; transporter associated with antigen processing.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
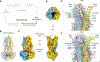

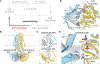

Update of
-
Nucleotide-dependent conformational changes direct peptide export by the transporter associated with antigen processing.bioRxiv [Preprint]. 2025 Jun 17:2025.06.12.659373. doi: 10.1101/2025.06.12.659373. bioRxiv. 2025. Update in: Immunity. 2025 Sep 9;58(9):2166-2175.e4. doi: 10.1016/j.immuni.2025.08.003. PMID: 40666987 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

