Modulating tumor collagen fiber alignment for enhanced lung cancer immunotherapy via inhaled RNA
- PMID: 40885724
- PMCID: PMC12398490
- DOI: 10.1038/s41467-025-63415-0
Modulating tumor collagen fiber alignment for enhanced lung cancer immunotherapy via inhaled RNA
Abstract
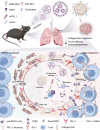
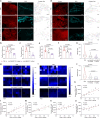
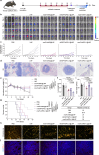
The clinical effectiveness of immunotherapies for lung cancers has been greatly hindered by the immune-excluded and immunosuppressive tumor microenvironment (TME) and limited pulmonary accessibility of therapeutics. Here, we develop an inhalable lipid nanoparticle (LNP) system that enables simultaneous delivery of mRNA encoding anti-discoidin domain receptor 1 (DDR1) single-chain variable fragments (mscFv) and siRNA targeting PD-L1 (siPD-L1) into pulmonary cancer cells. The secreted anti-DDR1 scFv blocks the binding of DDR1 extracellular domain to collagen, disrupting collagen fiber alignment and reducing tumor stiffness, thereby facilitating T cell infiltration. Meanwhile, PD-L1 silencing alleviates immunosuppression and preserves T cell cytotoxicity. In vivo results demonstrate that mscFv@LNP induces collagen fiber rearrangement and diminishes tumor stiffness. In both orthotopic and metastatic mouse models of lung cancer, inhalation of mscFv/siPD-L1@LNP promotes tumor regression and extends overall survival. This strategy could be broadly applicable to solid tumors and benefit other cancer immunotherapies by addressing the universally hostile TME involved in tumor progression.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Co-delivery of axitinib and PD-L1 siRNA for the synergism of vascular normalization and immune checkpoint inhibition to boost anticancer immunity.J Nanobiotechnology. 2025 Mar 10;23(1):194. doi: 10.1186/s12951-025-03170-y. J Nanobiotechnology. 2025. PMID: 40059141 Free PMC article.
-
Cancer-associated fibroblast-derived extracellular vesicles loaded with GLUT1 inhibitor synergize anti-PD-L1 to suppress tumor growth via degrading matrix stiffness and remodeling tumor microenvironment.J Control Release. 2025 Sep 10;385:113998. doi: 10.1016/j.jconrel.2025.113998. Epub 2025 Jul 1. J Control Release. 2025. PMID: 40609836
-
Interplay between tumor mutation burden and the tumor microenvironment predicts the prognosis of pan-cancer anti-PD-1/PD-L1 therapy.Front Immunol. 2025 Jul 24;16:1557461. doi: 10.3389/fimmu.2025.1557461. eCollection 2025. Front Immunol. 2025. PMID: 40777041 Free PMC article.
-
PD-L1 expression in advanced NSCLC: Insights into risk stratification and treatment selection from a systematic literature review.Lung Cancer. 2017 Oct;112:200-215. doi: 10.1016/j.lungcan.2017.08.005. Epub 2017 Aug 10. Lung Cancer. 2017. PMID: 29191596
-
The role of exosomal PD-L1 in NSCLC immunotherapy.Immunotherapy. 2025 Jul;17(10):715-725. doi: 10.1080/1750743X.2025.2539060. Epub 2025 Jul 30. Immunotherapy. 2025. PMID: 40735784 Review.
References
-
- Meric-Bernstam, F., Larkin, J., Tabernero, J. & Bonini, C. Enhancing anti-tumour efficacy with immunotherapy combinations. Lancet397, 1010–1022 (2021). - PubMed
-
- Kelly, P. N. The Cancer Immunotherapy Revolution. Science359, 1344–1345 (2018). - PubMed
-
- Chen, X. et al. Non-invasive activation of intratumoural gene editing for improved adoptive T-cell therapy in solid tumours. Nat. Nanotechnol.18, 933 (2023). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

