Selective right heart valve remodelling in a mouse model of carcinoid disease revealed by high-resolution episcopic microscopy
- PMID: 40885776
- PMCID: PMC12398488
- DOI: 10.1038/s41598-025-16064-8
Selective right heart valve remodelling in a mouse model of carcinoid disease revealed by high-resolution episcopic microscopy
Abstract
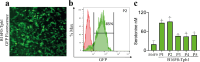
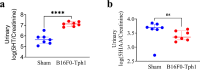
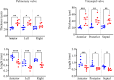
Carcinoid heart disease, a severe complication of neuroendocrine tumors, affects up to 50% of patients and is challenging to treat due to a limited understanding of its mechanisms. The disease is characterized by structural remodeling and thickening of the right heart valves, associated with elevated levels of serotonin (5-HT) released from tumor cells that have spread to the liver. Existing animal models have limitations as they either use mice with compromised immune systems or employ methods that don't consistently evaluate valve changes. We developed an improved experimental model by implanting syngeneic liver-targeted melanoma cells that were genetically engineered to produce 5-HT through the expression of the enzyme tryptophan hydroxylase type 1 (Tph1) in wild-type C57BL/6 mice. We introduced high-resolution episcopic microscopy (HREM) for comprehensive assessment of valve pathology and morphometry. Five weeks after implantation, mice exhibited increased 5-HT/creatinine urinary level ratios and HREM imaging showed selective thickening and structural remodeling of right heart valves (tricuspid and pulmonary), correlating with 5-HT/creatinine urinary level ratio, while left heart valves remained unaffected. Our data suggest that this non-immunosuppressed, right-heart valve restricted model reproduces key features of human carcinoid heart disease and, combined with HREM analysis, provides a valuable platform for studying disease mechanisms and testing potential therapies.
Keywords: Animal experimentation; Heart valves/pathology; High-resolution episcopic microscopy; Mice; Serotonin.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures





References
-
- Bhattacharyya, S., Toumpanakis, C., Chilkunda, D., Caplin, M. E. & Davar, J. Risk factors for the development and progression of carcinoid heart disease. Am. J. Cardiol.107, 1221–1226 (2011). - PubMed
-
- Delhomme, C. et al. Carcinoid heart disease in patients with midgut neuroendocrine tumours. J. Neuroendocrinol.35, e13262 (2023). - PubMed
-
- Suc, G., Cachier, A., Hentic, O., Bazire, B., Sannier, A., Delhomme, C., Nataf, P., Laschet, J., Deschamps, L., Garbarz, E., Ou, P., Caligiuri, G., Iung, B., Ruszniewski, P., de Mestier, L. & Arangalage, D. Management and outcomes of carcinoid heart disease with liver metastases of midgut neuroendocrine tumours. Heart (2023). - PubMed
-
- Suc, G., Cachier, A., Hascoet, S., Arangalage, D., Iung, B. & Urena, M. Transcatheter pulmonary valve replacement for the treatment of carcinoid heart disease. Rev. Esp. Cardiol. (Engl. Ed) (2024). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

