LZTR1 is a melanoma oncogene that promotes invasion and suppresses apoptosis
- PMID: 40885854
- PMCID: PMC12500468
- DOI: 10.1038/s41388-025-03538-2
LZTR1 is a melanoma oncogene that promotes invasion and suppresses apoptosis
Abstract
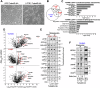
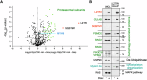
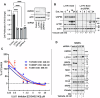
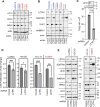
Leucine zipper like transcription regulator 1 (LZTR1) is amplified in acral melanomas, is required for melanocytes and melanoma cell proliferation, and it induces anchorage-independent growth, by yet unknown mechanisms. We therefore performed comprehensive studies to identify its activity in melanomas employing proximity biotinylation and co-immunoprecipitation combined with LC-MS/MS proteomics and molecular characterization. The results show that LZTR1 regulates the ubiquitin proteasome system in melanoma cells and also associates with actin-related proteins and actin cytoskeleton organization. Its downregulation suppresses the protective effect of the autophagy-initiating ULK1 and AMBRA1, regulators of normal cell survival and proliferation, and upregulates the sequestosome 1 (SQSTM1/p62), an autophagic cargo adapter which mediates selective degradation of ubiquitinated proteins. In contrast, overexpression of LZTR1 provides growth advantage under environmental stress, enhancing cell invasion, by activating ERBB3 receptor and its downstream targets PYK2 and SRC tyrosine kinases that regulate the cytoskeleton, actin organization, cell spreading, cell migration and adhesion. LZTR1 is a "safeguard" for melanoma cells under stress and its downregulation can be exploited for melanoma therapy.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics: We confirm that all methods were performed in accordance with the relevant guidelines and regulations. Research involving human participants was conducted in accordance with applicable laws, regulations, guidelines, and ethical principles such as those outlined in the Belmont Report. The research was approved by the Yale Human Research Protection Program (HRPP), registration number FWA00002571, which includes the Institutional Review Board (IRB), responsible for ensuring compliance with these standards and Yale’s institutional policies. Its goal is to: Ensure the rights and welfare of human research participants; Promote excellence in the conduct of research; and Advance scientific knowledge and quality research ( http://www.yale.edu/hrpp ). Informed consent was obtained from all the participants with IRB Protocol ID: 060900186. Title of Study: Yale Specimen and Data Repository. Investigator: Ruth Halaban.
Figures
References
-
- Nacak TG, Leptien K, Fellner D, Augustin HG, Kroll J. The BTB-kelch protein LZTR-1 is a novel Golgi protein that is degraded upon induction of apoptosis. J Biol Chem. 2006;281:5065–71. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

