Neutrophil expression of CD41/CD61 complex contributes to their adhesiveness in both healthy individuals and heart failure patients
- PMID: 40885916
- PMCID: PMC12398050
- DOI: 10.1186/s12865-025-00742-3
Neutrophil expression of CD41/CD61 complex contributes to their adhesiveness in both healthy individuals and heart failure patients
Abstract
Background: Neutrophils can release pro-inflammatory cytokines and neutrophil extracellular traps (NETs), leading to vascular thrombosis. Neutrophil-platelet interaction, a major component of thrombosis, is more pronounced in inflammatory pathologies, such as heart failure (HF). Recently, the platelet receptor CD41/CD61 (GPIIb/IIIa), known for its role in platelet adhesion and aggregation via its binding to fibrinogen, was found on the membrane of neutrophils from lung cancer patients. Our objectives were to determine CD41/CD61 expression on neutrophils and its role in healthy volunteers (HV) and acute decompensated heart failure patients (ADHF).
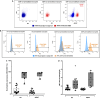
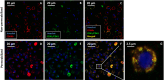
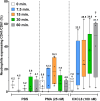
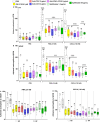
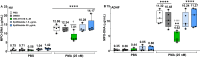
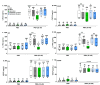
Results: Localization and expression of CD41/CD61 complex on isolated neutrophils were determined by flow cytometry and confocal microscopy. We assessed the CD41/CD61 role on neutrophil adhesion onto human extracellular matrix (hECM), NETosis and release of inflammatory cytokines. CD41/CD61 complex was intracellularly expressed in 80–90% of neutrophils, but only between 8 and 13% on their extracellular membrane. The CD41/CD61 complex plays a role in neutrophil adhesion onto hECM, since its blockade by an anti-CD41 monoclonal antibody or its antagonist, eptifibatide, reduced CXCL8 and PMA induced neutrophil adhesion from 52 to 100% in both HV and ADHF patients.
Conclusions: In summary, neutrophil specific CD41/CD61 expression contributes to neutrophil adhesiveness without affecting significantly the release of inflammatory cytokines and NETosis in both HV and ADHF patients.
Supplementary Information: The online version contains supplementary material available at 10.1186/s12865-025-00742-3.
Keywords: Adhesion; Heart failure; NETosis; Neutrophils.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study has been approved by the MHI’s Research Ethics Committee and performed with the accordance of the Declaration of Helsinki. Informed consent was obtained from all subjects prior to the study (Montreal, QC, Canada; ethics No. ICM #12-1374). Clinical trial number: not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Neutrophils are indispensable for adverse cardiac remodeling in heart failure.J Mol Cell Cardiol. 2024 Apr;189:1-11. doi: 10.1016/j.yjmcc.2024.02.005. Epub 2024 Feb 22. J Mol Cell Cardiol. 2024. PMID: 38387309 Free PMC article.
-
Role of microRNAs in neutrophil extracellular trap formation and prevention: Systematic narrative review.Mol Cell Probes. 2024 Dec;78:101986. doi: 10.1016/j.mcp.2024.101986. Epub 2024 Oct 13. Mol Cell Probes. 2024. PMID: 39389272
-
Exploring the involvement of serine proteases in neutrophil extracellular traps: a review of mechanisms and implications.Cell Death Dis. 2025 Jul 18;16(1):535. doi: 10.1038/s41419-025-07857-w. Cell Death Dis. 2025. PMID: 40681487 Free PMC article. Review.
-
Multiple cell death modalities and immune response in pulpitis.Int Endod J. 2025 Jan;58(1):111-127. doi: 10.1111/iej.14145. Epub 2024 Sep 10. Int Endod J. 2025. PMID: 39257034
-
NETosis and pyroptosis of immune cells in sepsis.J Transl Int Med. 2025 Aug 12;13(4):318-327. doi: 10.1515/jtim-2025-0035. eCollection 2025 Aug. J Transl Int Med. 2025. PMID: 40861069 Free PMC article.
References
-
- Sorop O, Heinonen I, van Kranenburg M, van de Wouw J, de Beer VJ, Nguyen ITN, et al. Multiple common comorbidities produce left ventricular diastolic dysfunction associated with coronary microvascular dysfunction, oxidative stress, and myocardial stiffening. Cardiovasc Res. 2018;114:954–64. - PMC - PubMed
-
- Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–71. - PubMed
-
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

