Cost-effectiveness of apixaban vs. aspirin for the reduction of thrombo-embolism in high-risk patients with device-detected atrial fibrillation: insights from the ARTESiA trial
- PMID: 40886070
- PMCID: PMC12448926
- DOI: 10.1093/europace/euaf195
Cost-effectiveness of apixaban vs. aspirin for the reduction of thrombo-embolism in high-risk patients with device-detected atrial fibrillation: insights from the ARTESiA trial
Abstract
Aims: Apixaban was superior to aspirin for the prevention of stroke or systemic embolism in participants with subclinical atrial fibrillation (SCAF) in the Apixaban for the Reduction of Thromboembolism in Patients With Device-Detected Subclinical Atrial Fibrillation trial. This was especially true for those with CHA2DS2-VASc score > 4. Understanding the cost-effectiveness of treating SCAF is important for decision-makers.
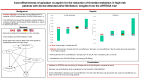
Methods and results: Canadian, UK, German, and US direct healthcare costs [in 2023 US dollars (USD)] were applied to hospitalized events (including strokes and bleeds) and study drugs for all participants with a CHA2DS2-VASc score > 4 to determine the mean cost per participant during the trial (mean follow-up 3.5 years). A daily cost of $0.63, $0.11, $2.26, and $6.06 for apixaban in Canada, the UK, Germany, and the USA was used. If in-trial results were not cost-saving (below $0), the prospective plan was to perform a lifetime cost-effectiveness analysis using a Markov model and a willingness-to-pay of 50 000 USD per quality-adjusted life year (QALY). After considering the cost of study medication and clinical events over 3.5 years, apixaban was dominant (cost-saving and more effective) in Canada (-$2301) and the UK (-$902) but cost more in Germany and the USA ($600 and $1990, respectively). Over a lifetime, treatment with apixaban produced a net gain of 0.107 QALYs, but with costs in both Germany ($2623 more) and the USA ($9110 more), yielding an incremental cost-effectiveness ratio of $24 514 per QALY for Germany and $85 140 for the USA.
Conclusion: In patients with SCAF and a CHA2DS2-VASc score > 4, apixaban is cost saving in Canada and the UK and cost-effective in Germany. Apixaban was not cost-effective in the USA under the base cost assumption but would be cost-effective at a daily cost of $4.35 and cost saving at $3.59.
Keywords: Apixaban; Cost-effectiveness; Stroke prevention; Subclinical atrial fibrillation.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: A.L., R.K.S., W.T., M.A., D.J.W., C.L., J.B.-M., and C.E.L. have no conflicts to declare. W.F.M. reports grant from Trimedica, consulting fees from Trimedica and AtriCure, and honoraria from iRhythm. R.D.L. reports consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Novo Nordisk, and Pfizer; speaking fees for AstraZeneca, Daiichi Sankyo, Novo Nordisk, and Pfizer; and institutional grant support from Amgen, Bristol Myers Squibb, GSK, Medtronic, Pfizer, and Sanofi. C.B.G. reports grants from Alnylam, Boehringer Ingelheim, Bristol Myers Squibb, US Food and Drug Administration, Janssen, Lilly, Novartis, Pfizer, Philips, and Roche; consulting fees from Alnylam, Boehringer Ingelheim, Bristol Myers Squibb, US Food and Drug Administration, Janssen, Lilly, Novartis, Pfizer, Philips, Roche, Merck, NIH, NephroSynergy, Novo Nordisk, REATA, and Veralox; honoraria from AbbVie, Abiomed, Amgen, Alnylam Pharmaceuticals, Anthos, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cardionomic, Cadrenal, CeleCor Therapeutics, Hengrui, Janssen, Medscape, Medtronic, Merck, NIH, NephroSynergy, Novo Nordisk, Novartis, Pfizer, Philips, REATA, Roche, and Veralox; data safety monitoring boards/advisory board from Bayer, Novartis, Novo Nordisk, Veralox, Cardionomic, Janssen, and Merck; and stock interests in Tenac.io. J.C.N. reports institutional research grants from the Novo Nordisk Foundation and the Danish Heart Foundation and being the executive editor for Europace. V.K. reports grants from Boston Scientific, ZOLL, Biotronik, Spire, and the National Institutes for Health; consulting fees from ZOLL; and honoraria from Abbott, Medtronic, ZOLL, and Biotronik. J.W.E. reports receiving consultant fees, travel support, and lecture fees from ZOLL Medical; speaker honoraria from Bayer Vital and Pfizer; and travel support from Bayer Vital and Boehringer Ingelheim. D.H.B. reports patient site and national leadership fees from the Population Health Research Institute for ARTESiA paid to their institution. D.A. reports speaker fees from Abbott, Amgen, Amarin, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Chiesi, GSK, MSD, Novartis, Novo Nordisk, Pfizer, Pharmacosmos, Philips, Roche Diagnostics, Sanofi, Takeda, and Vifor and grant support (to the institution) from BMS/Pfizer, Medtronic, Bayer, and Roche Diagnostics. S.H.H. has served as a consultant to Pfizer, Bristol Myers Squibb, Boehringer Ingelheim, and Sanofi. J.K. reports honoraria from Abbott, Biosense Webster, Biotronik, Boston Scientific, GE Healthcare, and Medtronic and served as a member of advisory boards for Biosense Webster, Boston Scientific, GE Healthcare, and Medtronic. A.J.C. reports personal fees from Acesion, Anthos, InCarda, Milestone, Daiichi Sankyo, Abbott, Boston Scientific, and Johnson & Johnson. C.S. reports consulting fees from Medtronic, Boston Scientific, and Biotronik and honoraria from MicroPort and Biotronik. M.R.G. reports consulting fees from Boston Scientific, Medtronic, and Abbott and speaker fees from Abbott and Boston Scientific. J.S.H. reports funding for the ARTESiA trial from BMS/Pfizer and Medtronic and for the ATLAS and LAAOS-4 trials from Boston Scientific; advisory board fees from Boston Scientific; speaker fees from BMS/Pfizer, Boston Scientific, and Medtronic; expert witness fees from Bayer; and data safety monitoring boards participation for the PRAETORIAN DFT trial from Boston Scientific.
Figures

References
-
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983–8. - PubMed
-
- Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263–72. - PubMed
-
- Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120–9. - PubMed
-
- Healey JS, Lopes RD, Granger CB, Alings M, Rivard L, McIntyre WF et al. Apixaban for stroke prevention in subclinical atrial fibrillation. N Engl J Med 2024;390:107–17. - PubMed
-
- Lopes RD, Granger CB, Wojdyla DM, McIntyre WF, Alings M, Mani T et al. Apixaban vs aspirin according to CHA2DS2-VASc score in subclinical atrial fibrillation: insights from ARTESiA. J Am Coll Cardiol 2024;84:354–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

