Skeletal muscle Rac1 mediates exercise training adaptations towards muscle glycogen resynthesis and protein synthesis
- PMID: 40886619
- PMCID: PMC12418856
- DOI: 10.1016/j.redox.2025.103844
Skeletal muscle Rac1 mediates exercise training adaptations towards muscle glycogen resynthesis and protein synthesis
Abstract
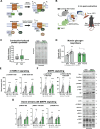
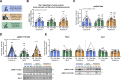
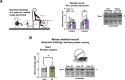
Long-term exercise training elicits tremendous health benefits; however, the molecular understanding is incomplete and identifying therapeutic targets has been challenging. Rho GTPases are among the most regulated groups of proteins after exercise in human skeletal muscle, yet, unexplored candidates for mediating the effects of exercise training. We found that the Rho GTPase Rac1 was activated acutely after multiple exercise modalities in human skeletal muscle. Loss of Rac1 specifically in muscle attenuated contraction-induced muscle protein synthesis, diminished improvements in running capacity, and prevented muscle hypertrophy after exercise training in mice. Additionally, Ncf1∗ mice revealed that Rac1 regulated glycogen resynthesis via a NOX2-dependent mechanism. Molecularly, Rac1 was required for contraction-induced p38MAPK signaling towards HSP27, MNK1, and CREB phosphorylation. In vivo muscle-targeted overexpression of a hyperactive Rac1-mutant elevated reactive oxidant species production during exercise but did not affect muscle mass. Using mass spectrometry-based proteomics, we found that loss or gain of Rac1 muscle protein affected pathways related to cytoskeleton organization, muscle adaptation, and large ribosomal subunits. Thus, skeletal muscle Rac1 mediates both molecular and functional adaptation to exercise training.
Keywords: Contraction; Exercise training; Glycogen; Metabolism; Muscle hypertrophy; Protein synthesis; Rac1; Skeletal muscle.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors of the current manuscript have no conflict of interest associated with the current manuscript.
Figures




References
-
- Blazev R., Carl C.S., Ng Y.-K., Molendijk J., Voldstedlund C.T., Zhao Y., Xiao D., Kueh A.J., Miotto P.M., Haynes V.R., Hardee J.P., Chung J.D., McNamara J.W., Qian H., Gregorevic P., Oakhill J.S., Herold M.J., Jensen T.E., Lisowski L., Lynch G.S., Dodd G.T., Watt M.J., Yang P., Kiens B., Richter E.A., Parker B.L. Phosphoproteomics of three exercise modalities identifies canonical signaling and C18ORF25 as an AMPK substrate regulating skeletal muscle function. Cell Metab. 2022;34:1561–1577.e9. doi: 10.1016/j.cmet.2022.07.003. - DOI - PubMed
-
- Hoffman N.J., Parker B.L., Chaudhuri R., Fisher-Wellman K.H., Kleinert M., Humphrey S.J., Yang P., Holliday M., Trefely S., Fazakerley D.J., Stöckli J., Burchfield J.G., Jensen T.E., Jothi R., Kiens B., Wojtaszewski J.F.P., Richter E.A., James D.E. Global phosphoproteomic analysis of human skeletal muscle reveals a network of exercise-regulated kinases and AMPK substrates. Cell Metab. 2015;22:948. doi: 10.1016/j.cmet.2015.10.004. - DOI - PMC - PubMed
-
- Sylow L., Jensen T.E., Kleinert M., Mouatt J.R., Maarbjerg S.J., Jeppesen J., Prats C., Chiu T.T., Boguslavsky S., Klip A., Schjerling P., Richter E.A. Rac1 is a novel regulator of contraction-stimulated glucose uptake in skeletal muscle. Diabetes. 2013;62:1139–1151. doi: 10.2337/db12-0491. - DOI - PMC - PubMed
-
- Sylow L., Nielsen I.L., Kleinert M., V Møller L.L., Ploug T., Schjerling P., Bilan P.J., Klip A., Jensen T.E., Richter E.A. Rac1 governs exercise-stimulated glucose uptake in skeletal muscle through regulation of GLUT4 translocation in mice. J. Physiol. 2016;17:4997–5008. doi: 10.1113/JP272039. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

