Suppression of adipocyte ABHD6 favors anti-inflammatory and adipogenic programs to preserve adipose tissue fitness in obesity
- PMID: 40886915
- PMCID: PMC12544160
- DOI: 10.1016/j.molmet.2025.102241
Suppression of adipocyte ABHD6 favors anti-inflammatory and adipogenic programs to preserve adipose tissue fitness in obesity
Abstract
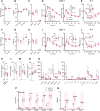
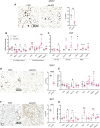
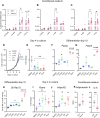
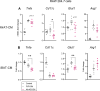
Some individuals exhibit metabolically healthy obesity, characterized by the expansion of white adipose tissue (WAT) without associated complications. The monoacylglycerol (MAG) hydrolase α/β-hydrolase domain-containing 6 (ABHD6) has been implicated in energy metabolism, with its global deletion conferring protection against obesity. However, the immunometabolic roles of adipocyte ABHD6 in WAT remodeling in response to nutri-stress and obesity are not known. Here, we demonstrate that in insulin resistant women, ABHD6 mRNA expression is elevated in visceral fat and positively correlates with obesity and metabolic dysregulation. ABHD6 expression is also elevated in the WATs of diet-induced obese and db/db mice. Although adipocyte-specific ABHD6 knockout (AA-KO) mice become obese under high-fat diet, they show higher plasma adiponectin, reduced circulating insulin and inflammatory markers, improved insulin sensitivity, and lower plasma and liver triglycerides. They also show enhanced insulin action in various tissues, but normal glucose tolerance. In addition, AA-KO mice display healthier and less inflamed expansion of visceral fat, with smaller adipocytes and higher stimulated lipolysis and fatty acid oxidation levels. Similar but less prominent phenotype was found in the subcutaneous and brown fat depots. Thus, adipocyte ABHD6 suppression prevents most of the metabolic and inflammatory complications of obesity, but not obesity per se. Mechanistically, this beneficial process involves a rise in MAG levels in mature adipocytes, and their secretion, resulting in a crosstalk among adipocytes, preadipocytes and macrophages in the adipose microenvironment. Elevated intracellular MAG causes PPARs activation in adipocytes, and MAG secreted from adipocytes curtails the inflammatory polarization of macrophages and promotes preadipocyte differentiation. Hence, adipocyte ABHD6 and MAG hydrolysis contribute to unhealthy WAT remodeling and expansion in obesity, and its suppression represents a candidate strategy to uncouple obesity from many of its immunometabolic complications.
Keywords: Adipose tissue; Inflammation; Insulin signaling; Macrophages; Monoacylglycerol; Obesity; PPARs; α/β-hydrolase domain-containing 6.
Copyright © 2025 The Authors. Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Marc Prentki reports financial support was provided by Canadian Institutes of Health Research. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Ghaben A.L., Scherer P.E. Adipogenesis and metabolic health. Nat Rev Mol Cell Biol. 2019;20(4):242–258. - PubMed
-
- Strissel K.J., Stancheva Z., Miyoshi H., Perfield J.W., 2nd, DeFuria J., Jick Z., et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56(12):2910–2918. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

