Glycyrrhizin alleviates contrast-induced acute kidney injury via inhibiting HMGB1-mediated renal tubular epithelial cells ferroptosis
- PMID: 40887103
- PMCID: PMC12404086
- DOI: 10.1080/0886022X.2025.2548613
Glycyrrhizin alleviates contrast-induced acute kidney injury via inhibiting HMGB1-mediated renal tubular epithelial cells ferroptosis
Abstract
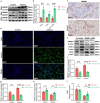
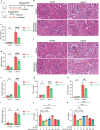
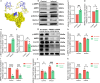
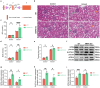
Contrast-induced acute kidney injury (CI-AKI) is the third leading cause of AKI, but there are no effective preventive or therapeutic measures in clinical practice. Glycyrrhizin, a bioactive compound isolated from the Glycyrrhiza glabra L., exhibits anti-inflammatory effects; however, the effects and mechanisms of glycyrrhizin on CI-AKI remain unknown. In present study, the effects of glycyrrhizin on renal dysfunction and tissue damage were evaluated in CI-AKI rats and mice. And the mechanisms were further investigated in iohexol treated renal tubular epithelial cells. Molecular docking and network pharmacology were used to discover the binding targets of glycyrrhizin and identify potential pathogenic pathway. Gene knockout mice and gene silencing cells were used to detect whether glycyrrhizin alleviated CI-AKI through target proteins mediated pathway. Results showed that both pretreatment and co-treatment with glycyrrhizin could alleviate iohexol-induced renal dysfunction and pathological damage in vivo. Similarly, glycyrrhizin could improve iohexol-induced decrease in cell viability of both HK-2 cells and primary mice renal tubular epithelial cells. Mechanistically, glycyrrhizin could directly bind to the active site of HMGB1, then blocking iohexol-induced ferroptosis of renal tubular epithelial cells. HMGB1 silencing was able to inhibit overactivation of AMPK/Beclin-1 axis during CI-AKI, and iohexol-downregulated protein expressions of GPX4 and SLC7A11 were reversed in kidneys of AMPK knockout mice. Comparable results were obtained in vitro with AICAR treatment. Our study is the first to demonstrate that glycyrrhizin exerts both protective and therapeutic effect on CI-AKI by inhibiting tubular epithelial cell ferroptosis via HMGB1/AMPK/Beclin-1 axis, providing a potential choice for treating CI-AKI.
Keywords: Contrast-induced AKI; HMGB1; ferroptosis; glycyrrhizin; renal tubular epithelial cells.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures




Similar articles
-
Salvianolic Acid B Attenuates Ferroptosis in Acute Kidney Injury by Targeting PRDX5.FASEB J. 2025 Jul 31;39(14):e70803. doi: 10.1096/fj.202500258RR. FASEB J. 2025. PMID: 40654183 Free PMC article.
-
SARS-CoV-2 ORF3a induces COVID-19-associated kidney injury through HMGB1-mediated cytokine production.mBio. 2024 Nov 13;15(11):e0230824. doi: 10.1128/mbio.02308-24. Epub 2024 Sep 30. mBio. 2024. PMID: 39345136 Free PMC article.
-
Hesperetin attenuates ischemia/reperfusion-induced acute kidney injury by regulating ferroptosis in mice.Ren Fail. 2025 Dec;47(1):2523573. doi: 10.1080/0886022X.2025.2523573. Epub 2025 Jun 29. Ren Fail. 2025. PMID: 40583332 Free PMC article.
-
Ferroptosis in acute kidney injury following crush syndrome: A novel target for treatment.J Adv Res. 2023 Dec;54:211-222. doi: 10.1016/j.jare.2023.01.016. Epub 2023 Jan 24. J Adv Res. 2023. PMID: 36702249 Free PMC article.
-
Diuretics for preventing and treating acute kidney injury.Cochrane Database Syst Rev. 2025 Jan 29;1(1):CD014937. doi: 10.1002/14651858.CD014937.pub2. Cochrane Database Syst Rev. 2025. PMID: 39878152
References
-
- Yang L, Xing G, Wang L, et al. Acute kidney injury in China: a cross-sectional survey. Lancet. 2015;386(10002):1465–1471. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
