Trifolium pratense-Derived Exosome Improved Serum Biochemical Parameters and Pancreatic Genes in STZ-Induced Diabetic Rats
- PMID: 40887321
- PMCID: PMC12399343
- DOI: 10.1002/edm2.70103
Trifolium pratense-Derived Exosome Improved Serum Biochemical Parameters and Pancreatic Genes in STZ-Induced Diabetic Rats
Abstract
Introduction: Plant-derived exosomes (PDEs) are promising nanotherapeutics for improving chronic diseases, such as diabetes mellitus. Trifolium pratense (TP) is a flowering herb with potent antioxidant and antidiabetic properties. The present study aimed to explore the diabetic-healing effects of TP-derived exosomes (TPDEs) in streptozotocin (STZ)-induced diabetic rats.
Methods: TPDEs were isolated using polyethylene glycol precipitation and serial centrifugation and characterised. STZ-induced diabetic rats were treated with TPDE doses (0, 100, 200, and 400 μg/kg) for 28 days. Biochemical factors (fasting blood sugar (FBS), insulin, C-peptide, total antioxidant capacity (TAC), and nitric oxide (NO)) were evaluated in serum samples. Also, the expression of PDX1, insulin, NGN3, and SIRT1 genes in pancreas tissues was assessed using real-time PCR.
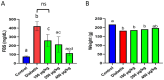
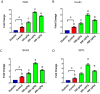
Results: TPDE treatment decreased the serum levels of FBS and NO while increasing c-peptide, insulin, and TAC levels. It also significantly enhanced the expression of insulin, PDX1, NGN3, and SIRT1 genes. TPDEs at doses of 100 to 200 μg/kg showed the most significant antidiabetic effects.
Conclusion: TPDEs significantly improved diabetes-induced alterations in serum insulin levels, antioxidant status, and pancreas-related gene expression. It can be considered a novel complementary treatment for diabetes.
Keywords: Trifolium pratense; diabetes; plant‐derived exosomes.
© 2025 The Author(s). Endocrinology, Diabetes & Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Antidiabetogenic action of Morus rubra L. leaf extract in streptozotocin-induced diabetic rats.J Pharm Pharmacol. 2010 Feb;62(2):247-55. doi: 10.1211/jpp.62.02.0013. J Pharm Pharmacol. 2010. PMID: 20487205
-
Rice bran extract attenuates cognitive impairment by enhancing pancreatic β-cell insulin secretion in STZ-induced diabetic rats targeting the PPARγ/PDX1 pathway.Metab Brain Dis. 2025 Jun 19;40(6):228. doi: 10.1007/s11011-025-01639-1. Metab Brain Dis. 2025. PMID: 40536613 Free PMC article.
-
Effect of Pelargonium graveolens on Glucose Metabolism in Streptozotocin- Induced Diabetic Rats.Cardiovasc Hematol Disord Drug Targets. 2022;22(1):18-26. doi: 10.2174/1871529X22666220216102243. Cardiovasc Hematol Disord Drug Targets. 2022. PMID: 35170422
-
Efficacy and safety of 'true' cinnamon (Cinnamomum zeylanicum) as a pharmaceutical agent in diabetes: a systematic review and meta-analysis.Diabet Med. 2012 Dec;29(12):1480-92. doi: 10.1111/j.1464-5491.2012.03718.x. Diabet Med. 2012. PMID: 22671971
-
Ayurvedic treatments for diabetes mellitus.Cochrane Database Syst Rev. 2011 Dec 7;2011(12):CD008288. doi: 10.1002/14651858.CD008288.pub2. Cochrane Database Syst Rev. 2011. PMID: 22161426 Free PMC article.
References
-
- Omar N., Nazirun N. N., Vijayam B., Wahab A. A., and Bahuri H. A., “Diabetes Subtypes Classification for Personalized Health Care: A Review,” Artificial Intelligence Review 56 (2023): 2697–2721, 10.1007/s10462-022-10202-8. - DOI
-
- Lima J. E., Moreira N. C., and Sakamoto‐Hojo E. T., “Mechanisms Underlying the Pathophysiology of Type 2 Diabetes: From Risk Factors to Oxidative Stress, Metabolic Dysfunction, and Hyperglycemia,” Mutation Research/Genetic Toxicology and Environmental Mutagenesis 874 (2022): 503437, 10.1016/j.mrgentox.2021.503437. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

