Arterial stiffness and vascular aging: mechanisms, prevention, and therapy
- PMID: 40887468
- PMCID: PMC12399776
- DOI: 10.1038/s41392-025-02346-0
Arterial stiffness and vascular aging: mechanisms, prevention, and therapy
Abstract
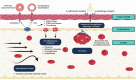
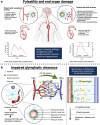
Cardiovascular diseases are the leading cause of morbidity and mortality worldwide. The central underlying mechanisms of cardiovascular diseases are vascular aging and associated arterial stiffness. Arterial stiffness is characterized by structural (e.g., tunica media calcification, alterations in vascular smooth muscle cells, and fibrosis) and functional (e.g., loss of Windkessel function, elevated pulse pressure, and development of isolated systolic hypertension) vascular changes that cause microvascular dysfunction and end-organ damage (e.g., heart failure, vascular dementia, hypertensive retinopathy, and chronic kidney disease). Current research indicates that arterial stiffness is an independent risk factor for cardiovascular diseases and represents a potential target for personalized prevention and therapeutic approaches. In this review, we summarize the pathophysiological mechanisms of vascular aging and arterial stiffness, outline the resulting end-organ damage, present different methods for the measurement of arterial stiffness, highlight the potential role of prevention and therapy, and provide future perspectives for arterial stiffness research. The purpose of this review is to provide a state-of-the-art interdisciplinary and translational approach to arterial stiffness, highlighting unique pathophysiological mechanisms (e.g., perivascular adipose tissue, extracellular vesicles), clinical relevance, and future directions.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






References
-
- Gaziano, T., Reddy, K. S., Paccaud, F., Horton, S. & Chaturvedi, V. Cardiovascular Disease. in Disease Control Priorities in Developing Countries. 2nd edition (The International Bank for Reconstruction and Development / The World Bank, 2006). - PubMed
-
- Cardiovascular diseases (CVDs). https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases... (2021).
-
- Fishbein, M. C. & Fishbein, G. A. Arteriosclerosis: facts and fancy. Cardiovasc. Pathol.24, 335–342 (2015). - PubMed
-
- Fishbein, G. A. & Fishbein, M. C. Arteriosclerosis: rethinking the current classification. Arch. Pathol. Lab. Med.133, 1309–1316 (2009). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

