The bacterial etiology and antimicrobial susceptibility of lower respiratory tract infections in Vietnam
- PMID: 40887577
- PMCID: PMC12400722
- DOI: 10.1186/s12941-025-00818-3
The bacterial etiology and antimicrobial susceptibility of lower respiratory tract infections in Vietnam
Abstract
Background: Lower respiratory tract infection (LRTI) remains the leading infectious cause of morbidity and mortality globally. Key bacterial pathogens include Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae, Escherichia coli, Staphylococcus aureus and Streptococcus pneumoniae. This study examined the prevalence and antimicrobial resistance patterns of major bacterial pathogens from community- and hospital-acquired LRTIs across six major hospitals in Vietnam.
Methods: Between January 2022 and May 2023, 1000 bacterial isolates were collected through an isolate-based surveillance. Species identification and antimicrobial susceptibility testing were performed by VITEK-2/Phoenix M50, with MICs determined by E-test or broth microdilution. Multiplex PCRs were used to detect common AMR genes.
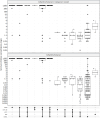
Results: A. baumannii (49.6%), P. aeruginosa (21%), K. pneumoniae (18.6%) were predominant, followed by S. aureus (6.7%), E. coli (3.9%) and S. pneumoniae (0.2%). Most isolates (94.4%) were identified from hospital-acquired cases. High prevalence of MDR and carbapenem resistance were identified in A. baumannii (96% and 95%), P. aeruginosa (56.7% and 57.1%), and K. pneumoniae (78% and 69.2%), respectively. Notably, resistance to ceftazidime-avibactam was detected in K. pneumoniae (34.3%), P. aeruginosa (29%), and E. coli (7.7%), while colistin resistance was found in K. pneumoniae (18.2%) and A. baumannii (2.8%). MRSA prevalence was 79.1%, though S. aureus remained susceptible to vancomycin, linezolid and ceftaroline. Most blaNDM-positive K. pneumoniae (62/71, 87.3%), E. coli (2/2, 100%), and P. aeruginosa (23/25, 85.2%) showed resistance to ceftazidime-avibactam. Whole genome sequencing revealed that the blaNDM-positive but ceftazidime-avibactam susceptible isolates (9 K. pneumoniae and 2 P. aeruginosa) carried truncated blaNDM. Overall, ceftazidime-avibactam was effective against K. pneumoniae, E. coli, and P. aeruginosa isolates carrying ESBL, ESBL and blaOXA-48, or ESBL and blaKPC. Alternatively, no detectable AMR genes were found in 35 ceftazidime-avibactam resistant P. aeruginosa isolates.
Conclusions: Carbapenem-resistant Gram-negative pathogens were predominant among hospital-acquired LRTIs in Vietnam, with notable resistance to ceftazidime-avibactam and colistin. The lack of effective treatment for A. baumannii remains a major concern. We found a strong correlation between AMR phenotype and genotype among K. pneumoniae and E. coli, supporting gene-based therapy to guide ceftazidime-avibactam use. However, the presence of disrupted blaNDM underscores the need to re-evaluate commercial PCR assays for carbapenemase detection.
Keywords: Antimicrobial resistance; Ceftazidime-avibactam; Lower respiratory tract infection; Nosocomial pneumonia.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethical approval of the study has been granted by the Institutional Review Boards at the six participating hospitals and Oxford Tropical Research Ethics Committee (approval number: 551 − 21). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Salam N, Jevtic M, Safiri S, Abdollahi M. Global burden of lower respiratory infections during the last three decades. https://vizhub.healthdata.org/gbd-compare/ - PMC - PubMed
-
- Troeger C, Blacker B, Khalil IA, Rao PC, Cao J, Zimsen SRM, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Infect Dis. 2018;18(11):1191–210. - PMC - PubMed
-
- Li Y, Nair H. Trends in the global burden of lower respiratory infections: the knowns and the unknowns. Lancet Infect Dis. 2022;22:1523–5. 10.1016/S1473-3099. - PubMed
-
- WHO reveals leading causes of death and disability worldwide. 2000–2019. https://www.who.int/news/item/09-12-2020-who-reveals-leading-causes-of-d.... Accessed 11 Aug 2023.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical